
Sample records for antioxidant-conjugated difluorodiarylidenyl piperidones
-
Synthesis and Lateral Root-Inducing Activity of N-Benzyl-3-Substituted-2-Piperidones
Tsukada, Hidetaka; Itamura, Tomoaki; Ishii, Rika; Taniguchi, Eiji; Kuwano, Eiichi
1999-01-01
Thirty N-benzyl-3-substituted-2-piperidones were synthesized, and their plant growth regulatory activity was evaluated by using a lettuce seedling test. Most of the compounds at 100 ppm caused lateral root formation. Of the series of compounds tested, N-benzyl-3-[1-hydroxy-1-(4-quinolyl)methyl]-2-piperidone (30) showed the highest activity. When 1ppm of compound 30 was supplied to seedlings, 29% of the primary roots formed at least one lateral root.
-
Hamdani, Afshan Mumtaz; Wani, Idrees Ahmed; Bhat, Naseer Ahmad; Siddiqi, Raushid Ahmad
2018-02-01
This study was undertaken to analyze the effect of conjugation of egg-white lysozyme with guar gum. Lysozyme is an antimicrobial polypeptide that can be used for food preservation. Its antibacterial activity is limited to gram positive bacteria. Conjugation with polysaccharides like guar gum may broaden its activity against gram negatives. Conjugate was developed through Maillard reaction. Assays carried out included sugar estimation, SDS-PAGE, GPC, color, FT-IR, DSC, thermal stability, solubility, emulsifying, foaming and antioxidant activity. In addition, antimicrobial activity of the conjugate was determined against two gram positive (Staphyllococcus aureus and Enterococcus) and two gram negative pathogens (E. coli and Salmonella). Results showed higher functional properties of lysozyme-guar gum conjugate. The antioxidant properties increased from 2.02-35.80% (Inhibition of DPPH) and 1.65-4.93AAE/g (reducing power) upon guar gum conjugation. Conjugate significantly inhibited gram negative bacteria and the antibacterial activity also increased significantly against gram positive pathogens. Copyright © 2017 Elsevier Ltd. All rights reserved.
-
Synthesis, structure, antioxidant activity, and water solubility of trolox ion conjugates
Directory of Open Access Journals (Sweden)
Yuliya V. Yushkova
2018-01-01
Full Text Available The interaction of trolox with ammonia, alkylamines of different classes, and amino derivatives of heterocyclic compounds, including nitroxyl radicals and alkaloids, led to the production of ammonium salts called ion conjugates (ICs. Five ICs were characterised by X-ray diffraction. This is the first time a wide range of ICs were made from trolox with amines, and ESI-MS data demonstrated they have the potential to generate pseudomolecular [(A−B+ + H]+ ions. For all obtained trolox ICs, a significant increase (1–3 orders of magnitude in water solubility was achieved while retaining high antioxidant activity. ICs synthesised from two biologically active fragments may be used to create polyfunctional agents with varying solubility and bioavailability. Keywords: Trolox, Amines, Ion conjugates, Antioxidants, Mass-spectrometry
-
Hu, Qiaobin; Wang, Taoran; Zhou, Mingyong; Xue, Jingyi; Luo, Yangchao
2016-07-27
The major objective of this work was to develop a green and facile process to prepare gallic acid-chitosan conjugate and comprehensively evaluate the physicochemical properties and biological activities of an as-prepared water-soluble chitosan derivative. A free-radical-induced grafting approach using an ascorbic acid-hydrogen peroxide redox pair was adopted. The obtained conjugate was characterized by Fourier transform infrared spectroscopy, UV-vis, X-ray diffraction, and pKa analysis. The antioxidant activities were evaluated by 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2'-azino-bis(3-ethylbenzothiazoline-6)-sulphonic acid (ABTS), reducing power, and oxygen-radical antioxidant-capacity assays. The results showed that the mass ratio of gallic acid to chitosan played a vital role in determining the grafting degree and ζ potential of the conjugates, with the ratio of 0.5:1 being the optimal ratio that resulted in the highest grafting degree. The antioxidant assays demonstrated that conjugation significantly improved the antioxidant activities, being dramatically higher than that of free chitosan. It was notable that the DPPH- and ABTS-scavenging activities of conjugate at 0.4 mg/mL reached the same level as the free gallic acid at the equivalent concentration. Our study demonstrated a green and facile synthesis approach to preparing a novel water-soluble chitosan derivative that may have promising potentials in the food industry.
-
Synthesis and evaluation of the antioxidative potential of minoxidil-polyamine conjugates.
Hadjipavlou-Litina, Dimitra; Magoulas, George E; Bariamis, Stavros E; Tsimali, Zinovia; Avgoustakis, Konstantinos; Kontogiorgis, Christos A; Athanassopoulos, Constantinos M; Papaioannou, Dionissios
2013-07-01
A series of conjugates (MNX-CO-PA) of minoxidil (MNX) with the polyamines (PAs) putrescine (PUT), spermidine (SPD) and spermine (SPM) as well as dopamine were produced through activation of MNX with N,N'-carbonyldiimidazole, followed by reaction with dopamine or selectively protected PAs and acid-mediated deprotection. These conjugates together with conjugates of the general type MNX-PA or PA-MNX-PA, readily produced using literature protocols, were tested as antioxidants. The most potent inhibitors of lipid peroxidation were the conjugates MNX-SPM (2, 94%), SPM-MNX-SPM (4, 94%) and MNX-N(4)-SPD (7, 91%) and MNX (91%). The most powerful lipoxygenase (LOX) inhibitors were MNX (IC50 = 20 μM) and the conjugates MNX-N(8)-SPD (9, IC50 = 22.1 μM), MNX-CO-dopamine (11, IC50 = 28 μM) and MNX-N(1)-SPD (8, IC50 = 30 μM). The most interesting conjugates 2, MNX-CO-PUT (5), 8 and 11 as well as MNX were generally found to exhibit weaker (22-36.5%) or no (conjugate 8) anti-inflammatory activity than indomethacin (47%) with the exception of MNX which showed almost equal potency (49%) to indomethacin. The cytocompatibility of conjugates and MNX at the highest concentration of 100 μM showed a survival percentage of 87-107%, with the exception of conjugates with SPM (compound 2) and MNX-CO-SPM (6), which showed considerable cytotoxicity (survival percentage 8-14%). Molecular docking studies were carried on conjugate 9 and the parent compound MNX and were found to be in accordance with our experimental biological results. Copyright © 2013 Elsevier Masson SAS. All rights reserved.
-
Directory of Open Access Journals (Sweden)
Ortensia I. Parisi
2017-08-01
Full Text Available Background: Melanins are high molecular weight pigments responsible for the mammalian skin and hair colour and play a key role in skin protection from UV radiation; however, their overproduction and excessive accumulation lead to pigmentation problems including melasma, freckles, uneven colouring, and age spots. Therefore, the modulation of melanin synthesis represents a critical issue in medicine and cosmetology. In the present study, an innovative polymeric antioxidant to be used as skin whitening agent is developed by the conjugation of dextran with rosmarinic acid. Methods: Dextran-rosmarinic acid conjugates (DEX-RA were synthesized in a one-pot method starting from Origanum vulgare aqueous leaf extract and dextran. The total polyphenol content and the antioxidant activity were assessed by Folin-Ciocalteau assay and 2,2-diphenyl-1-picrylhydrazyl radical (DPPH and bleaching tests, respectively. The efficacy of DEX-RA was evaluated by inhibition of tyrosinase activity, in vitro diffusion and stability studies and in vivo studies. The biocompatibility of the conjugates was investigated by 3-[4,5-Dimethylthiaoly]-2,5-diphenyltetrazoliumbromide (MTT and EPISKIN™ model. Results: Efficacy and safety studies confirmed the antioxidant and tyrosinase inhibitory activities and the biocompatibility of the synthesized conjugates. Conclusion: The polymeric conjugates, comparing to the free antioxidant, show a long-lasting efficacy combined to an enhanced stability resulting in an improved performance of the cosmetic formulations prepared using this innovative whitening agent as a bioactive ingredient.
-
Sun, Yuanxia; Hayakawa, Shigeru; Ogawa, Masahiro; Izumori, Ken
2005-12-28
Protein-sugar conjugates generated in nonenzymatic glycation of alpha-lactalbumin (LA) with rare sugars [D-allose (All) and D-psicose (Psi)] and alimentary sugars as controls [D-glucose (Glc) and D-fructose (Fru)] were qualitatively determined by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Mass spectra revealed that the extent of glycation at lysine residues on LA with D-aldose molecules was very much higher than that of glycation with d-ketose molecules. To identify the specific site of glycation, the peptide mapping was established from protease V8 digestion, using a combination of computational cutting of proteins and MALDI-TOF-MS. As compared to peptide mapping, three and seven glycation sites were located in the primary structure of LA-ketose and LA-aldose conjugates, respectively. On the other hand, the antioxidant activities of protein-sugar conjugates and their peptic hydrolysates were investigated by 1,1-diphenyl-2-picrylhydrazyl radical scavenging method. The antioxidant activities of proteins/peptides glycated with rare sugars were significantly higher than those modified with the control sugars. The results indicated that the glycation degree and position were not markedly different between rare sugar and corresponding control sugar, but the antioxidant properties of protein and its hydrolysate were significantly enhanced by modifying with rare sugar.
-
Directory of Open Access Journals (Sweden)
Xican Li
2018-03-01
Full Text Available α-Viniferin and caraphenol A, the two oligostilbenes, have the sole difference of the presence or absence of an exocyclic double bond at the π-π conjugative site. In this study, the antioxidant capacity and relevant mechanisms for α-viniferin and caraphenol A were comparatively explored using spectrophotometry, UV-visible spectral analysis, and electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UPLC–ESI–Q–TOF–MS/MS analysis. The spectrophotometric results suggested that caraphenol A always gave lower IC50 values than α-viniferin in cupric ion-reducing antioxidant capacity assay, ferric-reducing antioxidant power assay, 1,1-diphenyl-2-picryl-hydrazl radical (DPPH•-scavenging, and 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical-scavenging assays. In UV-visible spectra analysis, caraphenol A was observed to show enhanced peaks at 250–350 nm when mixed with Fe2+, but α-viniferin exhibited no similar effects. UPLC–ESI–Q–TOF–MS/MS analysis revealed that α-viniferin mixed with DPPH• produced radical adduct formation (RAF peak (m/z = 1070–1072. We conclude that the antioxidant action of α-viniferin and caraphenol A may involve both redox-mediated mechanisms (especially electron transfer and H+-transfer and non-redox-mediated mechanisms (including Fe2+-chelating or RAF. The π-π conjugation of the exocyclic double bond in caraphenol A can greatly enhance the redox-mediated antioxidant mechanisms and partially promote the Fe2+-chelating mechanism. This makes caraphenol A far superior to α-viniferin in total antioxidant levels.
-
Directory of Open Access Journals (Sweden)
Maximiliano Martínez-Cifuentes
2016-12-01
Full Text Available In this work, a computational study of a series of N-substitued-4-piperidones curcumin analogues is presented. The molecular structure of the neutral molecules and their radical anions, as well as their reactivity, are investigated. N-substituents include methyl and benzyl groups, while substituents on the aromatic rings cover electron-donor and electron-acceptor groups. Substitutions at the nitrogen atom do not significantly affect the geometry and frontier molecular orbitals (FMO energies of these molecules. On the other hand, substituents on the aromatic rings modify the distribution of FMO. In addition, they influence the capability of these molecules to attach an additional electron, which was studied through adiabatic (AEA and vertical electron affinities (VEA, as well as vertical detachment energy (VDE. To study electrophilic properties of these structures, local reactivity indices, such as Fukui (f+ and Parr (P+ functions, were calculated, and show the influence of the aromatic rings substituents on the reactivity of α,β-unsaturated ketones towards nucleophilic attack. This study has potential implications for the design of curcumin analogues based on a 4-piperidone core with desired reactivity.
-
Hashemi Gahruie, Hadi; Niakousari, Mehrdad
2017-11-01
Polymeric antioxidants such as Catechinaldehyde Polycondensates, Catechin-acelaldehydepolycondensates, Flavonoid-grafted chitosan fibers, Ferulate hydrogel, Dextran ferulate hydrogel, Starch-quercetin conjugate, Gallic acid- and Caffeic acid-functionalized chitosan, Gallic acid - chitosan conjugate, Poly(rutin), Gallic acid grafted chitosan, Dextran-Catechin Conjugate belong to biological macromolecules. These kinds of compounds have stronger antioxidant potential and pharmacokinetic activities, as compared to similar low molecular weight preservatives. Most of these compounds sources are either antioxidants with low molecules polymerization, or polymers conjugation such as synthetic or natural preservatives. Additives are well known as being an important ingredient of food products due to their strong preservative potential. Many researchers and industries attempt to find synthesize materials with the same antioxidant potential and higher stability than the similar compounds with low molecular weight. Recently, macromolecular antioxidants have received wide attention as food additives and dietary supplements in functional foods. It seems that the main usage of these compounds is in the food packaging industry. Most of these compounds have strong antioxidant, antimicrobial, cell viability and enzymatic inhibitory properties. Copyright © 2017 Elsevier B.V. All rights reserved.
-
Directory of Open Access Journals (Sweden)
Yanqing Liu
2017-02-01
Full Text Available A highly enantioselective organocatalytic Wolff rearrangement–amidation–Michael–hemiaminalization stepwise reaction is described involving a cyclic 2-diazo-1,3-diketone, primary amine and α,β-unsaturated aldehyde. Product stereocontrol can be achieved by adjusting the sequence of steps in this one-pot multicomponent reaction. This approach was used to synthesize various optically active spirocyclic piperidones with three stereogenic centers and multiple functional groups in good yields up to 76%, moderate diastereoselectivities of up to 80:20 and high enantioselectivities up to 97%.
-
Sun, Liping; Zhang, Huilin; Zhuang, Yongliang
2012-02-01
The soluble phenolic compounds of rambutan peels (RP) were extracted by microwave-assisted extraction (MAE) and the operating parameters were optimized. The optimal conditions obtained were ethanol concentration of 80.85%, extraction time of 58.39 s, and the ratio of liquid to solid of 24.51:1. The soluble phenolic content by MAE was 213.76 mg GAE/g DW. The free, soluble conjugate, and insoluble-boaund phenolic compounds were prepared by alkaline hydrolysis, and the contents of 3 fractions were 185.12, 27.98 and 9.37 mg GAE/g DW, respectively. The contents of syringic acid and p-coumaric acid were high in the free fraction, showing 16.86 and 19.44 mg/g DW, and the soluble conjugate and insoluble-bound phenolics were mainly composed of gallic acid and caffeic acid. Furthermore, the antioxidant activities of 3 fractions were evaluated in 5 model systems. Results indicated that the free fraction had high antioxidant activities, compared with the soluble conjugate and insoluble-bound fractions. © 2012 Institute of Food Technologists®
-
Fullerene-biomolecule conjugates and their biomedicinal applications.
Yang, Xinlin; Ebrahimi, Ali; Li, Jie; Cui, Quanjun
2014-01-01
Fullerenes are among the strongest antioxidants and are characterized as "radical sponges." The research on biomedicinal applications of fullerenes has achieved significant progress since the landmark publication by Friedman et al in 1993. Fullerene-biomolecule conjugates have become an important area of research during the past 2 decades. By a thorough literature search, we attempt to update the information about the synthesis of different types of fullerene-biomolecule conjugates, including fullerene-containing amino acids and peptides, oligonucleotides, sugars, and esters. Moreover, we also discuss in this review recently reported data on the biological and pharmaceutical utilities of these compounds and some other fullerene derivatives of biomedical importance. While within the fullerene-biomolecule conjugates, in which fullerene may act as both an antioxidant and a carrier, specific targeting biomolecules conjugated to fullerene will undoubtedly strengthen the delivery of functional fullerenes to sites of clinical interest.
-
Weilbeer, Claudia; Sickert, Marcel; Naumov, Sergei; Schneider, Christoph
2017-01-12
We disclose herein the first enantioselective aza-Diels-Alder reaction of β-alkyl-substituted vinylketene silyl-O,O-acetals and imines furnishing a broad range of optically highly enriched 4-alkyl-substituted 2-piperidones. As a catalyst for this one-pot reaction we employed a chiral phosphoric acid which effects a vinylogous Mannich reaction directly followed by ring-closure to the lactam. Subsequent fully diastereoselective transformations including hydrogenation, enolate alkylation, and lactam alkylation/reduction processes converted the cycloadducts into various highly substituted piperidines of great utility for the synthesis of natural products and medicinally active compounds. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
-
Siddalingappa, Basavaraj; Benson, Heather A. E.; Brown, David H.; Batty, Kevin T.; Chen, Yan
2015-01-01
Resveratrol is naturally occurring phytochemical with diverse biological activities such as chemoprevention, anti-inflammatory, anti-cancer, anti-oxidant. But undergoes rapid metabolism in the body (half life 0.13h). Hence Polymer conjugation utilizing different chemical linkers and polymer compositions was investigated for enhanced pharmacokinetic profile of resveratrol. Ester conjugates such as α-methoxy-ω-carboxylic acid poly(ethylene glycol) succinylamide resveratrol (MeO-PEGN-Succ-RSV) (2 and 20 kDa); MeO-PEG succinyl ester resveratrol (MeO-PEGO-Succ-RSV) (2 kDa); α-methoxy poly(ethylene glycol)-co-polylactide succinyl ester resveratrol (MeO-PEG-PLAO-Succ-RSV) (2 and 6.6kDa) were prepared by carbodiimide coupling reactions. Resveratrol-PEG ethers (2 and 5 kDa) were synthesized by alkali-mediated etherification. All polymer conjugates were fully characterized in vitro and the pharmacokinetic profile of selected conjugates was characterized in rats. Buffer and plasma stability of conjugates was dependent on polymer hydrophobicity, aggregation behavior and PEG corona, with MeO-PEG-PLAO-Succ-RSV (2 kDa) showing a 3h half-life in rat plasma in vitro. Polymer conjugates irrespective of linker chemistry protected resveratrol against metabolism in vitro. MeO-PEG-PLAO-Succ-RSV (2 kDa), Resveratrol-PEG ether (2 and 5 kDa) displayed improved pharmacokinetic profiles with significantly higher plasma area under curve (AUC), slower clearance and smaller volume of distribution, compared to resveratrol. PMID:25799413
-
Abdelhedi, Ola; Mora, Leticia; Jemil, Ines; Jridi, Mourad; Toldrá, Fidel; Nasri, Moncef; Nasri, Rim
2017-09-01
The effect of ultrasound (US) pre-treatment on the evolution of Maillard reaction (MR), induced between low molecular weight (LMW) peptides and sucrose, was studied. LMW peptides (reaction, a marked decrease in pH values, coupled to the increase in colour of the Maillard reaction products (MRPs), were recorded. In addition, after sonication, the glycation degree was significantly enhanced in Esperase-derived peptides/sucrose conjugates (p<0.05). Moreover, results showed that thermal heating, particularly after US treatment, reduced the bitter taste and enhanced the antioxidant capacities of the resulting conjugates. Hence, it could be concluded that US leads to efficient mixing of sugar-protein solution and efficient heat/mass transfer, contributing to increase the MR rate. Copyright © 2017 Elsevier Ltd. All rights reserved.
-
Functional properties of nisin–carbohydrate conjugates formed by radiation induced Maillard reaction
International Nuclear Information System (INIS)
Muppalla, Shobita R.; Sonavale, Rahul; Chawla, Surinder P.; Sharma, Arun
2012-01-01
Nisin–carbohydrate conjugates were prepared by irradiating nisin either with glucose or dextran. Increase in browning and formation of intermediate products was observed with a concomitant decrease in free amino and reducing sugar groups indicating occurrence of the Maillard reaction catalyzed by irradiation. Nisin–carbohydrate conjugates showed a broad spectrum antibacterial activity against Gram negative bacteria (Escherichia coli, Pseudomonas fluorescence) as well as Gram positive bacteria (Staphylococcus aureus, Bacillus cereus). Results of antioxidant assays, including that of DPPH radical-scavenging activity and reducing power, showed that the nisin–dextran conjugates possessed better antioxidant potential than nisin–glucose conjugate. These results suggested that it was possible to enhance the functional properties of nisin by preparing radiation induced conjugates suitable for application in food industry. - Highlights: ► Nisin–carbohydrate conjugates were prepared using radiation induced Maillard reaction. ► Conjugation of nisin with dextran/glucose resulted in improvement of antibacterial spectrum. ► Conjugates of nisin with dextran/glucose had significant radical scavenging activity.
-
Functional properties of nisin-carbohydrate conjugates formed by radiation induced Maillard reaction
Muppalla, Shobita R.; Sonavale, Rahul; Chawla, Surinder P.; Sharma, Arun
2012-12-01
Nisin-carbohydrate conjugates were prepared by irradiating nisin either with glucose or dextran. Increase in browning and formation of intermediate products was observed with a concomitant decrease in free amino and reducing sugar groups indicating occurrence of the Maillard reaction catalyzed by irradiation. Nisin-carbohydrate conjugates showed a broad spectrum antibacterial activity against Gram negative bacteria (Escherichia coli, Pseudomonas fluorescence) as well as Gram positive bacteria (Staphylococcus aureus, Bacillus cereus). Results of antioxidant assays, including that of DPPH radical-scavenging activity and reducing power, showed that the nisin-dextran conjugates possessed better antioxidant potential than nisin-glucose conjugate. These results suggested that it was possible to enhance the functional properties of nisin by preparing radiation induced conjugates suitable for application in food industry.
-
Catalytic asymmetric synthesis of the alkaloid (+)-myrtine
Pizzuti, Maria Gabriefla; Minnaard, Adriaan J.; Feringa, Ben L.
2008-01-01
A new protocol for the asymmetric synthesis of trans-2,6-disubstituted-4-piperidones has been developed using a catalytic enantioselective conjugate addition reaction in combination with a diastereoselective lithiation-substitution sequence; an efficient synthesis of (+)-myrtine has been achieved
-
Fullerene–biomolecule conjugates and their biomedicinal applications
Directory of Open Access Journals (Sweden)
Yang X
2013-12-01
Full Text Available Xinlin Yang,1 Ali Ebrahimi,1 Jie Li,1,2 Quanjun Cui11Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, University of Virginia School of Medicine, Charlottesville, VA, USA; 2School of Materials Science, Beijing Institute of Technology, Beijing, People's Republic of ChinaAbstract: Fullerenes are among the strongest antioxidants and are characterized as "radical sponges." The research on biomedicinal applications of fullerenes has achieved significant progress since the landmark publication by Friedman et al in 1993. Fullerene–biomolecule conjugates have become an important area of research during the past 2 decades. By a thorough literature search, we attempt to update the information about the synthesis of different types of fullerene–biomolecule conjugates, including fullerene-containing amino acids and peptides, oligonucleotides, sugars, and esters. Moreover, we also discuss in this review recently reported data on the biological and pharmaceutical utilities of these compounds and some other fullerene derivatives of biomedical importance. While within the fullerene–biomolecule conjugates, in which fullerene may act as both an antioxidant and a carrier, specific targeting biomolecules conjugated to fullerene will undoubtedly strengthen the delivery of functional fullerenes to sites of clinical interest.Keywords: fullerene, amino acid, peptide, oligonucleotide, sugar, ester
-
Phonsatta, Natthaporn; Deetae, Pawinee; Luangpituksa, Pairoj; Grajeda-Iglesias, Claudia; Figueroa-Espinoza, Maria Cruz; Le Comte, Jérôme; Villeneuve, Pierre; Decker, Eric A; Visessanguan, Wonnop; Panya, Atikorn
2017-08-30
The addition of antioxidants is one of the strategies to inhibit lipid oxidation, a major cause of lipid deterioration in foods leading to rancidity development and nutritional losses. However, several studies have been reported that conventional antioxidant assays, e.g., TPC, ABTS, FRAP, and ORAC could not predict antioxidant performance in several foods. This study aimed to investigate the performance of two recently developed assays, e.g., the conjugated autoxidizable triene (CAT) and the apolar radical-initiated conjugated autoxidizable triene (ApoCAT) assays to predict the antioxidant effectiveness of gallic acid and its esters in selected food models in comparison with the conventional antioxidant assays. The results indicated that the polarities of the antioxidants have a strong impact on antioxidant activities. In addition, different oxidant locations demonstrated by the CAT and ApoCAT assays influenced the overall antioxidant performances of the antioxidants with different polarities. To validate the predictability of the assays, the antioxidative performance of gallic acid and its alkyl esters was investigated in oil-in-water (O/W) emulsions, bulk soybean oils, and roasted peanuts as the lipid food models. The results showed that only the ApoCAT assay could be able to predict the antioxidative performances in O/W emulsions regardless of the antioxidant polarities. This study demonstrated that the relevance of antioxidant assays to food models was strongly dependent on physical similarities between the tested assays and the food structure matrices.
-
Simoni, Elena; Bergamini, Christian; Fato, Romana; Tarozzi, Andrea; Bains, Sandip; Motterlini, Roberto; Cavalli, Andrea; Bolognesi, Maria Laura; Minarini, Anna; Hrelia, Patrizia; Lenaz, Giorgio; Rosini, Michela; Melchiorre, Carlo
2010-10-14
Mitochondria-directed antioxidants 2-5 were designed by conjugating curcumin congeners with different polyamine motifs as vehicle tools. The conjugates emerged as efficient antioxidants in mitochondria and fibroblasts and also exerted a protecting role through heme oxygenase-1 activation. Notably, the insertion of a polyamine function into the curcumin-like moiety allowed an efficient intracellular uptake and mitochondria targeting. It also resulted in a significant decrease in the cytotoxicity effects. 2-5 are therefore promising molecules for neuroprotectant lead discovery.
-
Saranya, T S; Rajan, V K; Biswas, Raja; Jayakumar, R; Sathianarayanan, S
2018-04-15
Curcumin is a diaryl heptanoid of curcuminoids class obtained from Curcuma longa. It possesses various biological activities like anti-inflammatory, hypoglycemic, antioxidant, wound-healing, and antimicrobial activities. Chitosan is a biocompatible, biodegradable and non-toxic natural polymer which enhances the adhesive property of the skin. Chemical conjugation will leads to sustained release action and to enhance the bioavailability. This study aims to synthesis and characterize biocompatible curcumin conjugated chitosan microspheres for bio-medical applications. The Schiff base reaction was carried out for the preparation of curcumin conjugated chitosan by microwave method and it was characterised using FTIR and NMR. Curcumin conjugated chitosan microspheres (CCCMs) were prepared by wet milling solvent evaporation method. SEM analysis showed these CCCMs were 2-5μm spherical particles. The antibacterial activities of the prepared CCCMs were studied against Staphylococcus aureus and Escherichia coli, the zone of inhibition was 28mm and 23mm respectively. Antioxidant activity of the prepared CCCMs was also studied by DPPH and H 2 O 2 method it showed IC 50 esteem value of 216μg/ml and 228μg/ml, and anti-inflammatory activity results showed that CCCMs having IC 50 value of 45μg/ml. The results conclude that the CCCMs having a good antibacterial, antioxidant and anti-inflammatory activities. This, the prepared CCCMs have potential application in preventing skin infections. Copyright © 2017. Published by Elsevier B.V.
-
Biotransformation of Flavonoid Conjugates with Fatty Acids and Evaluations of Their Functionalities
Directory of Open Access Journals (Sweden)
Cynthia Q. Sun
2017-11-01
Full Text Available Enzymatic conjugation with fatty acids including omega-3 polyunsaturated fatty acids (ω-3 PUFAs derived from fish oil to three citrus fruit-derived flavonoids: grapefruit extract, naringin, and neohesperidin dihydrochalcone were investigated. The conversions were achieved over 85% under the catalysis of lipase Novozyme 435 in acetone at 45°C at semi-preparative scale. The conjugates were purified via solvent partition and silica gel chromatography and achieved 90–98% in purity. The NMR analysis of the conjugates confirmed that the fatty acid carbon chain was linked onto the primary –OH group on the glucose moiety of the flavonoids. The purified flavonoid conjugates alongside their original flavonoids were analyzed for antioxidant activities via 2,2-diphenyl-1-picrylhydrazyl scavenging assay, and anti-peroxidation test via peroxide values measured during a 1-week fish oil storage trial. Vascular endothelial growth factor (VEGF assay was conducted with 1, 10, and 100 μM of naringin and grapefruits and their conjugates, respectively, and total VEGF levels were measured at 24 and 48 h, respectively, using ELISA and dot blot analysis. The results from these functionality experiments demonstrated that flavonoid FA conjugates have at least comparable (if not higher antioxidant activity, anti-peroxidation activity, and anti-angiogenic activity.
-
Novel flavonolignan hybrid antioxidants: From enzymatic preparation to molecular rationalization.
Vavříková, Eva; Křen, Vladimír; Jezova-Kalachova, Lubica; Biler, Michal; Chantemargue, Benjamin; Pyszková, Michaela; Riva, Sergio; Kuzma, Marek; Valentová, Kateřina; Ulrichová, Jitka; Vrba, Jiří; Trouillas, Patrick; Vacek, Jan
2017-02-15
A series of antioxidants was designed and synthesized based on conjugation of the hepatoprotective flavonolignan silybin with l-ascorbic acid, trolox alcohol or tyrosol via a C 12 aliphatic linker. These hybrid molecules were prepared from 12-vinyl dodecanedioate-23-O-silybin using the enzymatic regioselective acylation procedure with Novozym 435 (lipase B) or with lipase PS. Voltammetric analyses showed that the silybin-ascorbic acid conjugate exhibited excellent electron donating ability, in comparison to the other conjugates. Free radical scavenging, antioxidant activities and cytoprotective action were evaluated. The silybin-ascorbic acid hybrid exhibited the best activities (IC 50 = 30.2 μM) in terms of lipid peroxidation inhibition. The promising protective action of the conjugate against lipid peroxidation can be attributed to modulated electron transfer abilities of both the silybin and ascorbate moieties, but also to the hydrophobic C 12 linker facilitating membrane insertion. This was supported experimentally and theoretically by density functional theory (DFT) and molecular dynamics (MD) calculations. The results presented here can be used in the further development of novel multipotent antioxidants and cytoprotective agents, in particular for substances acting at an aqueous/lipid interface. Copyright © 2016 Elsevier Masson SAS. All rights reserved.
-
Comparison of the antioxidant effects of carnosic acid and synthetic antioxidants on tara seed oil.
Li, Zhan-Jun; Yang, Feng-Jian; Yang, Lei; Zu, Yuan-Gang
2018-04-04
In the present study, tara seed oil was obtained by supercritical fluid extraction and used to investigate the antioxidant strength of carnosic acid (CA) compared with conventional synthetic antioxidants. The antioxidants were added to the tara seed oil at 0.2 mg of antioxidant per gram of oil. The samples were then submitted to at 60 °C 15 days for an accelerated oxidation process, with samples taken regularly for analysis. After oxidation, the samples were analyzed to determine the peroxide value, thiobarbituric acid reactive substances, conjugated diene content, and free fatty acid content. CA was investigated at three purity levels (CA20, CA60, CA99), and compared with three synthetic antioxidants (butylatedhydroxyanisole, butylatedhydroxytoluene, and tert-butylhydroquinone). The oxidation indicators showed that CA was a strong antioxidant compared to the synthetic antioxidants. The antioxidant activities decreased in the order: tert-butylhydroquinone > CA99 > CA60 > CA20 > butylatedhydroxyanisole > butylatedhydroxytoluene. These results show that CA could be used to replace synthetic antioxidants in oil products, and should be safer for human consumption and the environment.
-
Antipova, Anna S; Zelikina, Darya V; Shumilina, Elena A; Semenova, Maria G
2016-10-01
The present work is focused on the structural transformation of the complexes, formed between covalent conjugate (sodium caseinate + maltodextrin) and an equimass mixture of the polyunsaturated lipids (PULs): (soy phosphatidylcholine + triglycerides of flaxseed oil) stabilized by a plant antioxidant (an essential oil of clove buds), in the simulated conditions of the gastrointestinal tract. The conjugate was used here as a food-grade delivery vehicle for the PULs. The release of these PULs at each stage of the simulated digestion was estimated. Copyright © 2016 Elsevier Ltd. All rights reserved.
-
Pérez-López, Usue; Pinzino, Calogero; Quartacci, Mike Frank; Ranieri, Annamaria; Sgherri, Cristina
2014-12-10
Differently colored lettuce (Lactuca sativa L.) cultivars (green, green/red, and red) were studied to correlate their phenolic composition with their antioxidant kinetic behavior. Electron paramagnetic resonance (EPR) was employed to monitor decay kinetics of 1,1-diphenyl-2-picrylhydrazyl (DPPH(•)), which allowed the identification of three differently paced antioxidants. The results showed that as long as lettuce had higher red pigmentation, the hydrophilic antioxidant capacity increased together with the contents in free and conjugated phenolic acids, free and conjugated flavonoids, and anthocyanins. EPR allowed the identification of slow-rate antioxidants in green and green/red cultivars, intermediate-rate antioxidants in green, green/red, and red cultivars, and fast-rate antioxidants in green/red and red cultivars. At present, the different kinetic behaviors cannot be attributed to a specific antioxidant, but it is suggested that the flavonoid quercetin accounted for the majority of the intermediate-rate antioxidants, whereas the anthocyanins accounted for the majority of the fast-rate antioxidants.
-
Chrysin-piperazine conjugates as antioxidant and anticancer agents
Czech Academy of Sciences Publication Activity Database
Patel, Rahul V.; Mistry, B.M.; Syed, R.; Rathi, A.K.; Lee, Y. J.; Sung, J.S.; Shinf, H.S.; Keum, Y.S.
2016-01-01
Roč. 88, JUN 10 (2016), s. 166-177 ISSN 0928-0987 R&D Projects: GA MŠk(CZ) LO1204 Institutional support: RVO:61389030 Keywords : Chrysin * Antioxidant * Piperazines Subject RIV: EB - Genetics ; Molecular Biology Impact factor: 3.756, year: 2016
-
Evaluation of antioxidant and antimutagenic potential of Justicia ...
African Journals Online (AJOL)
sunny t
total phenolic content of the extracts is measured in terms of gallic acid equivalents .... The reducing activity on superoxide anion (O2. −*. ) was measured by modified ..... conjugates: Synthesis, antioxidant and antimutagenic attributes. Food.
-
Antioxidant activity of banana flavonoids.
Vijayakumar, S; Presannakumar, G; Vijayalakshmi, N R
2008-06-01
The antioxidant activity of flavonoids from banana (Musa paradisiaca) was studied in rats fed normal as well as high fat diets. Concentrations of peroxidation products namely malondialdehyde, hydroperoxides and conjugated diens were significantly decreased whereas the activities of catalase and superoxide dismutase were enhanced significantly. Concentrations of glutathione were also elevated in the treated animals.
-
Directory of Open Access Journals (Sweden)
Ru Song
2016-06-01
Full Text Available The antioxidative, antibacterial, and food functional properties of the half-fin anchovy hydrolysates (HAHp-glucose conjugates formed by Maillard reaction (MR were investigated, respectively. Results of sugar and amino acid contents loss rates, browning index, and molecular weight distribution indicated that the initial pH of HAHp played an important role in the process of MR between HAHp and glucose. HAHp-glucose Maillard reaction products (HAHp-G MRPs demonstrated enhanced antioxidative activities of reducing power and scavenging DPPH radicals compared to control groups. HAHp-G MRPs produced from the condition of pH 9.6 displayed the strongest reducing power. The excellent scavenging activity on DPPH radicals was found for HAHp(5.6-G MRPs which was produced at pH 5.6. Additionally, HAHp(5.6-G MRPs showed variable antibacterial activities against Escherichia coli, Pseudomonas fluorescens, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Bacillus megaterium, and Sarcina lutea, with the MIC values ranging from 8.3 to 16.7 μg/mL. Result of scanning electron microscopy (SEM on E. coli suggested that HAHp(5.6-G MRPs exhibited antibacterial activity by destroying the cell integrity through membrane permeabilization. Moreover, HAHp(5.6-G MRPs had excellent foaming ability and stability at alkaline conditions of pH 8.0, and showed emulsion properties at acidic pH 4.0. These results suggested that specific HAHp-G MRPs should be promising functional ingredients used in foods.
-
Song, Ru; Yang, Peiyu; Wei, Rongbian; Ruan, Guanqiang
2016-06-20
The antioxidative, antibacterial, and food functional properties of the half-fin anchovy hydrolysates (HAHp)-glucose conjugates formed by Maillard reaction (MR) were investigated, respectively. Results of sugar and amino acid contents loss rates, browning index, and molecular weight distribution indicated that the initial pH of HAHp played an important role in the process of MR between HAHp and glucose. HAHp-glucose Maillard reaction products (HAHp-G MRPs) demonstrated enhanced antioxidative activities of reducing power and scavenging DPPH radicals compared to control groups. HAHp-G MRPs produced from the condition of pH 9.6 displayed the strongest reducing power. The excellent scavenging activity on DPPH radicals was found for HAHp(5.6)-G MRPs which was produced at pH 5.6. Additionally, HAHp(5.6)-G MRPs showed variable antibacterial activities against Escherichia coli, Pseudomonas fluorescens, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Bacillus megaterium, and Sarcina lutea, with the MIC values ranging from 8.3 to 16.7 μg/mL. Result of scanning electron microscopy (SEM) on E. coli suggested that HAHp(5.6)-G MRPs exhibited antibacterial activity by destroying the cell integrity through membrane permeabilization. Moreover, HAHp(5.6)-G MRPs had excellent foaming ability and stability at alkaline conditions of pH 8.0, and showed emulsion properties at acidic pH 4.0. These results suggested that specific HAHp-G MRPs should be promising functional ingredients used in foods.
-
Altunkaya, Arzu; Gökmen, Vural; Skibsted, Leif H
2016-01-01
Influence of pH on the antioxidant activities of combinations of lettuce extract (LE) with quercetin (QC), green tea extract (GTE) or grape seed extract (GSE) was investigated for both reduction of Fremy's salt in aqueous solution using direct electron spin resonance (ESR) spectroscopy and in L-α-phosphatidylcholine liposome peroxidation assay measured following formation of conjugated dienes. All examined phenolic antioxidants showed increasing radical scavenging effect with increasing pH values by using both methods. QC, GTE and GSE acted synergistically in combination with LE against oxidation of peroxidating liposomes and with QC showing the largest effect. The pH dependent increase of the antioxidant activity of the phenols is due to an increase of their electron-donating ability upon deprotonation and to their stabilization in alkaline solutions leading to polymerization reaction. Such polymerization reactions of polyphenolic antioxidants can form new oxidizable -OH moieties in their polymeric products resulting in a higher radical scavenging activity. Copyright © 2015 Elsevier Ltd. All rights reserved.
-
Molecular Strategies for Targeting Antioxidants to Mitochondria: Therapeutic Implications
2015-01-01
Abstract Mitochondrial function and specifically its implication in cellular redox/oxidative balance is fundamental in controlling the life and death of cells, and has been implicated in a wide range of human pathologies. In this context, mitochondrial therapeutics, particularly those involving mitochondria-targeted antioxidants, have attracted increasing interest as potentially effective therapies for several human diseases. For the past 10 years, great progress has been made in the development and functional testing of molecules that specifically target mitochondria, and there has been special focus on compounds with antioxidant properties. In this review, we will discuss several such strategies, including molecules conjugated with lipophilic cations (e.g., triphenylphosphonium) or rhodamine, conjugates of plant alkaloids, amino-acid- and peptide-based compounds, and liposomes. This area has several major challenges that need to be confronted. Apart from antioxidants and other redox active molecules, current research aims at developing compounds that are capable of modulating other mitochondria-controlled processes, such as apoptosis and autophagy. Multiple chemically different molecular strategies have been developed as delivery tools that offer broad opportunities for mitochondrial manipulation. Additional studies, and particularly in vivo approaches under physiologically relevant conditions, are necessary to confirm the clinical usefulness of these molecules. Antioxid. Redox Signal. 22, 686–729. PMID:25546574
-
Czech Academy of Sciences Publication Activity Database
Domnina, N. S.; Sergeeva, O. Yu.; Koroleva, A. N.; Rakitina, O. V.; Dobrun, L. A.; Filippov, Sergey K.; Mikhailova, M. E.; Lezov, A. V.
2010-01-01
Roč. 52, č. 9 (2010), s. 900-906 ISSN 0965-545X Institutional research plan: CEZ:AV0Z40500505 Keywords : antioxidant * conjugates * light scattering Subject RIV: CD - Macromolecular Chemistry Impact factor: 0.659, year: 2010
-
Grasso, Giuseppa Ida; Bellia, Francesco; Arena, Giuseppe; Satriano, Cristina; Vecchio, Graziella; Rizzarelli, Enrico
2017-07-28
Increasing evidence is accumulating, showing that neurodegenerative disorders are somehow associated with the toxicity of amyloid aggregates, metal ion dyshomeostasis as well as with products generated by oxidative stress. Within the biological oxidation products, acrolein does have a prominent role. A promising strategy to deal with the above neurogenerative disorders is to use multi-functions bio-molecules. Herein, we show how a class of bio-conjugates takes advantage of the antiaggregating, antioxidant and antiglycating properties of trehalose and carnosine. Their ability to sequester acrolein and to inhibit both self- and metal-induced aggregation is here reported. The copper(II) coordination properties of a new trehalose-carnosine conjugate and the relative antioxidant effects have also been investigated. Copyright © 2017 Elsevier Masson SAS. All rights reserved.
-
Liu, Jun; Bai, Ruyu; Liu, Yunpeng; Zhang, Xin; Kan, Juan; Jin, Changhai
2018-02-01
In recent years, several medicinal plants have been demonstrated as valuable resources of naturally occurring polysaccharide-polyphenolic conjugates. For the first time, this article introduces recent advances of polysaccharide-polyphenolic conjugates isolated from different medicinal plants. The isolation, purification, structural characterization and biological activities of polysaccharide-polyphenolic conjugates are introduced in details. In general, polysaccharide-polyphenolic conjugates can be isolated by hot water or alkaline extraction followed by purification through anion exchange chromatography or gel filtration chromatography. The structures of conjugates are usually characterized by chemical composition analysis, UV-vis, Fourier-transform infrared and nuclear magnetic resonance spectroscopy. Moreover, polysaccharide-polyphenolic conjugates exhibit several biological activities including anticoagulant, antioxidant, radioprotective, anti-platelet, antitussive and bronchodilatory effects. Therefore, polysaccharide-polyphenolic conjugates isolated from medicinal plants are certain to have a bright prospect in the field of food and pharmaceutics. Copyright © 2017 Elsevier B.V. All rights reserved.
-
Succinobucol’s New Coat — Conjugation with Steroids to Alter Its Drug Effect and Bioavailability
Directory of Open Access Journals (Sweden)
Satu Ikonen
2011-11-01
Full Text Available Synthesis, detailed structural characterization (X-ray, NMR, MS, IR, elemental analysis, and studies of toxicity, antioxidant activity and bioavailability of unique potent anti-atherosclerotic succinobucol-steroid conjugates are reported. The conjugates consist of, on one side, the therapeutically important drug succinobucol ([4-{2,6-di-tert-butyl-4-[(1-{[3-tert-butyl-4-hydroxy-5-(propan-2-ylphenyl]sulfanyl}ethylsulfanyl]phenoxy}-4-oxo-butanoic acid] possessing an antioxidant and anti-inflammatory activity, and on the other side, plant stanol/sterols (stigmastanol, β-sitosterol and stigmasterol possessing an ability to lower the blood cholesterol level. A cholesterol-succinobucol prodrug was also prepared in order to enhance the absorption of succinobucol through the intestinal membrane into the organism and to target the drug into the place of lipid metabolism—The enterohepatic circulation system. Their low toxicity towards mice fibroblasts at maximal concentrations, their antioxidant activity, comparable or even higher than that of ascorbic acid as determined by direct quenching of the DPPH radical, and their potential for significantly altering total and LDL cholesterol levels, suggest that these conjugates merit further studies in the treatment of cardiovascular or other related diseases. A brief discussion of succinobucol’s ability to quench the radicals, supported with a computational model of the electrostatic potential mapped on the electron density surface of the drug, is also presented.
-
Fernández-Arroyo, Salvador; Herranz-López, María; Beltrán-Debón, Raúl; Borrás-Linares, Isabel; Barrajón-Catalán, Enrique; Joven, Jorge; Fernández-Gutiérrez, Alberto; Segura-Carretero, Antonio; Micol, Vicente
2012-10-01
The aqueous extracts of Hibiscus sabdariffa have been commonly used in folk medicine. Nevertheless, the compounds or metabolites responsible for its healthy effects have not yet been identified. The major metabolites present in rat plasma after acute ingestion of a polyphenol-enriched Hibiscus sabdariffa extract were characterized and quantified in order to study their bioavailability. The antioxidant status of the plasma samples was also measured through several complementary antioxidant techniques. High-performance liquid chromatography coupled to time-of-flight mass spectrometry (HPLC-ESI-TOF-MS) was used for the bioavailability study. The antioxidant status was measured by ferric reducing ability of plasma method, thiobarbituric acid reactive substances assay, and superoxide dismutase activity assay. Seventeen polyphenols and metabolites have been detected and quantified. Eleven of these compounds were metabolites. Although phenolic acids were found in plasma without any modification in their structures, most flavonols were found as quercetin or kaempferol glucuronide conjugates. Flavonol glucuronide conjugates, which show longer half-life elimination values, are proposed to contribute to the observed lipid peroxidation inhibitory activity in the cellular membranes. By contrast, phenolic acids appear to exert their antioxidant activity through ferric ion reduction and superoxide scavenging at shorter times. We propose that flavonol-conjugated forms (quercetin and kaempferol) may be the compounds responsible for the observed antioxidant effects and contribute to the healthy effects of H. sabdariffa polyphenolic extract. © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
-
Directory of Open Access Journals (Sweden)
L. E. A. Camargo
Full Text Available Abstract The antioxidant and anticandidal activities of leaves obtained from Camellia sinensis by non-fermentation (green and white teas, semi-fermentation (red tea and fermentation method (black tea were investigated. It was evaluated the total phenolic content by Folin-Ciocalteau assay; antioxidant capacities were evaluated in vitro using DPPH and ABTS radicals, hypochlorous acid and superoxide anion scavenger assays, induced hemolysis, lipid peroxidation by conjugated diene formation and myeloperoxidase activity. Anticandidal activity was performed on three strains of Candida spp. The results showed that non-fermented teas have a higher concentration of phenolic compounds, and then presented the best inhibitory activity of AAPH-induced hemolysis, the best inhibition of conjugated diene formation and more pronounced antioxidant activity in all tests. The highest anticandidal activity was obtained from fermented tea, followed by non-fermented tea. These results indicate that the antioxidant activity demonstrated has no direct relation with the anticandidal activity.
-
International Nuclear Information System (INIS)
Hruska, F.E.; Berger, Maurice; Cadet, Jean; Remin, Mieczyslaw
1985-01-01
γ-Irradiation of oxygen-free, aqueous solutions of 2'-deoxythymidine in the presence of the organic nitroxide free radical, 2,2,6,6-tetramethyl-1,4-piperidone-N-oxyl (TAN) leads to a complex mixture of products in which the TAN moiety is linked to the C5 or C6 position of a 5,6-saturated thymine ring. Extensive sup(1)H and sup(13)C nmr data are provided for the eight TAN-dT adducts which are produced in the largest amounts. The results show that the conformational properties of the sugar moiety are dependent on the point of attachment of the TAN group and the configuration of the standard thymine ring
-
Bifunctional phenolic-choline conjugates as anti-oxidants and acetylcholinesterase inhibitors
Czech Academy of Sciences Publication Activity Database
Šebestík, Jaroslav; Marques, S. M.; Falé, P. L.; Santos, S.; Arduíno, D. M.; Cardoso, S. M.; Oliveira, S. M.; Serralheiro, M. L. M.; Santos, A. M.
Roč. 26, č. 4 (485), s. 497 ISSN 1475-6366 Institutional research plan: CEZ:AV0Z40550506 Keywords : acetylcholinesterase inhibitors * antioxidants * hybrid ligands * anti-neurodegeneratives * Alzheimer´s disease Subject RIV: CC - Organic Chemistry
-
Widening and Elaboration of Consecutive Research into Therapeutic Antioxidant Enzyme Derivatives
Directory of Open Access Journals (Sweden)
Alexander V. Maksimenko
2016-01-01
Full Text Available Undiminishing actuality of enzyme modification for therapeutic purposes has been confirmed by application of modified enzymes in clinical practice and numerous research data on them. Intravenous injection of the superoxide dismutase-chondroitin sulfate-catalase (SOD-CHS-CAT conjugate in preventive and medicative regimes in rats with endotoxin shock induced with a lipopolysaccharide bolus has demonstrated that antioxidant agents not only effectively prevent damage caused by oxidative stress (as believed previously but also can be used for antioxidative stress therapy. The results obtained emphasize the importance of investigation into the pathogenesis of vascular damage and the role of oxidative stress in it. The effects of intravenous medicative injection of SOD-CHS-CAT in a rat model of endotoxin shock have demonstrated a variety in the activity of this conjugate in addition to prevention of NO conversion in peroxynitrite upon interaction with O2∙- superoxide radical. Together with the literature data, these findings offer a prospect for the study of NO-independent therapeutic effects of SOD-CHS-CAT, implying the importance of a better insight into the mechanisms of the conjugate activity in modeled cardiovascular damage involving vasoactive agents other than NO.
-
Metabolism of tert-butylhydroquinone to S-substituted conjugates in the male fischer 344 rat
Peters, M.M.C.G.; Lau, S.S.; Dulik, D.; Murphy, D.; Ommen, B. van; Bladeren, P.J. van; Monks, T.J.
1996-01-01
tert-Butyl-4-hydroxyanisole (BHA) and its demethylated analog, tert- butyl-hydroquinone (TBHQ), are antioxidants used in food. Both BHA and TBHQ have been shown to promote kidney and bladder carcinogenesis in the rat. We have previously demonstrated that glutathione (GSH) conjugates of a variety of
-
Role of paramagnetic polyconjugated clusters in lignin antioxidant activity (in vitro)
International Nuclear Information System (INIS)
Dizhbite, T; Ponomarenko, J; Andersone, A; Dobele, G; Lauberts, M; Krasilnikova, J; Telysheva, G; Mironova-Ulmane, N
2012-01-01
Using physico-chemical methods (EPR, SEC, Py-GC/MS and UV/VIS spectroscopy) and wet chemical analysis, the characteristics of 6 hardwood lignins in terms of functionality, molecular weight and composition of lignin substructures were determined and considered together with the results of DPPH., ABTS. + and O 2 . − antioxidant assays with the aim to understand the relationships governing antioxidant properties of lignin. The strong positive linear correlation between lignin antioxidant capacity in the three assays used and the extent of conjugation of paramagnetic polyconjugated clusters in lignin macromolecules was found. The biological activity of the most active alkaline lignins was assessed by in vitro experiment with human blood.
-
Directory of Open Access Journals (Sweden)
Cesar Garcias-Morales
2015-10-01
Full Text Available This paper reports the synthesis of a series of piperidones 1–8 by the Mannich reaction and analysis of their structures and conformations in solution by NMR and mass spectrometry. The six-membered rings in 2,4,6,8-tetraphenyl-3,7-diazabicyclo[3.3.1]nonan-9-ones, compounds 1 and 2, adopt a chair–boat conformation, while those in 2,4-diphenyl-3-azabicyclo[3.3.1]nonan-9-ones, compounds 3–8, adopt a chair–chair conformation because of stereoelectronic effects. These stereoelectronic effects were analyzed by the 1JC–H coupling constants, which were measured in the 13C satellites of the 1H NMR spectra obtained with the hetero-dqf pulse sequence. In the solid state, these stereoelectronic effects were investigated by measurement of X-ray diffraction data, the molecular geometry (torsional bond angles and bond distances, and inter- and intramolecular interactions, and by natural bond orbital analysis, which was performed using density functional theory at the ωB97XD/6311++G(d,p level. We found that one of the main factors influencing the conformational stability of 3–8 is the interaction between the lone-pair electrons of nitrogen and the antibonding sigma orbital of C(7–Heq (nN→σ*C–H(7eq, a type of hyperconjugative interaction.
-
Chrysin-piperazine conjugates as antioxidant and anticancer agents.
Patel, Rahul V; Mistry, Bhupendra; Syed, Riyaz; Rathi, Anuj K; Lee, Yoo-Jung; Sung, Jung-Suk; Shinf, Han-Seung; Keum, Young-Soo
2016-06-10
Synthesis of 7-(4-bromobutoxy)-5-hydroxy-2-phenyl-4H-chromen-4-one intermediate treating chrysin with 1,4-dibromobutane facilitated combination of chrysin with a wide range of piperazine moieties which were equipped via reacting the corresponding amines with bis(2-chloroethyl)amine hydrochloride in diethylene glycol monomethyl ether solvent. Free radical scavenging potential of prepared products was analyzed in vitro adopting DPPH and ABTS bioassay in addition to the evaluation of in vitro anticancer efficacies against cervical cancer cell lines (HeLa and CaSki) and an ovarian cancer cell line SK-OV-3 using SRB assay. Bearable toxicity of 7a-w was examined employing Madin-Darby canine kidney (MDCK) cell line. In addition, cytotoxic nature of the presented compounds was inspected utilizing Human bone marrow derived mesenchymal stem cells (hBM-MSCs). Overall, 7a-w indicated remarkable antioxidant power in scavenging DPPH(·) and ABTS(·+), particularly analogs 7f, 7j, 7k, 7l, 7n, 7q, 7v, 7w have shown promising free radical scavenging activity. Analogs 7j and 7o are identified to be highly active candidates against HeLa and CaSki cell lines, whereas 7h and 7l along with 7j proved to be very sensitive towards ovarian cancer cell line SKOV-3. None of the newly prepared scaffolds showed cytotoxic nature toward hBM-MSCs cells. From the structure-activity point of view, nature and position of the electron withdrawing and electron donating functional groups on the piperazine core may contribute to the anticipated antioxidant and anticancer action. Different spectroscopic techniques (FT-IR, (1)H NMR, (13)C NMR, Mass) and elemental analysis (CHN) were utilized to confirm the desired structure of final compounds. Copyright © 2016 Elsevier B.V. All rights reserved.
-
Electrochemical Behavior and Antioxidant and Prooxidant Activity of Natural Phenolics
Directory of Open Access Journals (Sweden)
Marija Todorović
2007-10-01
Full Text Available We have investigated the electrochemical oxidation of a number natural phenolics (salicylic acid, m-hydroxybenzoic acid, p-hydroxybenzoic acid, protocatechuic acid, o-coumaric acid, m-coumaric acid, p-coumaric acid, caffeic acid, quercetin and rutin using cyclic voltammetry. The antioxidant properties of these compounds were also studied. A structural analysis of the tested phenolics suggests that multiple OH substitution and conjugation are important determinants of the free radical scavenging activity and electrochemical behavior. Compounds with low oxidation potentials (Epa lower than 0.45 showed antioxidant activity, whereas compounds with high Epa values (>0.45 act as prooxidants.
-
Abel, Ezra Elumalai; John Poonga, Preetam Raj; Panicker, Shirly George
2016-01-01
This study was aimed to determine the effectiveness of synthesized gold nanoparticles of an ethnobotanically and medicinally important plant species Cassia tora against colon cancer cells and to find its antibacterial and antioxidant activities. In order to improve the bioavailability of C. tora, we synthesized gold nanoparticles through green synthesis, by simple mixing and stirring of C. tora leaf powder and tetrachloroauric acid (HAuCl4) solution which gave a dispersion of gold nanoparticles conjugate with C. tora secondary metabolites (SMs) with characteristic surface plasmon resonance. It was characterized by Fourier transform infrared spectroscopy, zeta sizer, zeta potential and transmission electron microscopy. Antibacterial activity was carried out for gold nanoparticles conjugated with C. tora SMs, using well-diffusion method. The MTT assay for cell viability and markers such as catalase, nitric oxide and lipid peroxidation was predictable to confirm the cytotoxicity and antioxidant properties. The treatment of gold nanoparticles conjugated with C. tora SMs on Col320 cells showed reduction in the cell viability through MTT assay, and it also significantly suppressed the release of H2O2, LPO and NO production in a dose-dependent manner. C. tora SMs conjugate gold nanoparticles showed enhanced bioavailability, antioxidant and anticancer effect against colon cancer cell line (Col320).
-
Saluk-Juszczak, Joanna; Pawlaczyk, Izabela; Olas, Beata; Kołodziejczyk, Joanna; Ponczek, Michal; Nowak, Pawel; Tsirigotis-Wołoszczak, Marta; Wachowicz, Barbara; Gancarz, Roman
2010-12-01
Lots of plants belonging to Asteraceae family are very popular in folk medicine in Poland. These plants are also known as being rich in acidic polysaccharides, due to the presence of hexuronic acids or its derivatives. Our preliminary experiments have shown that the extract from Conyza canadensis L. possesses various biological activity, including antiplatelet, antiocoagulant and antioxidant properties. The aim of our study was to assess if macromolecular glycoconjugates from selected herbal plants of Asteraceae family: Achillea millefolium L., Arnica montana L., Echinacea purpurea L., Solidago virgaurea L., Chamomilla recutita (L.) Rauschert., and Conyza canadensis L. protect platelet proteins against nitrative and oxidative damage induced by peroxynitrite, which is responsible for oxidative/nitrative modifications of platelet proteins: the formation of 3-nitrotyrosine and carbonyl groups. These modifications may lead to changes of blood platelet functions and can have pathological consequences. The role of these different medicinal plants in the defence against oxidative/nitrative stress in human platelets is still unknown, therefore the oxidative damage to platelet proteins induced by peroxynitrite and protectory effects of tested conjugates by the estimation of carbonyl group level and nitrotyrosine formation (a marker of protein nitration) were studied in vitro. The antioxidative properties of the polyphenolic-polysaccharide conjugates from selected tested medicinal plants were also compared with the action of a well characterized antioxidative commercial polyphenol - resveratrol (3,4',5-trihydroxystilbene). The obtained results demonstrate that the compounds from herbal plants: A. millefolium, A. montana, E. purpurea, C. recutita, S. virgaurea, possess antioxidative properties and protect platelet proteins against peroxynitrite toxicity in vitro, similar to the glycoconjugates from C. canadensis. However, in the comparative studies, the polyphenolic
-
Mezza, Gabriela N; Borgarello, Ana V; Grosso, Nelson R; Fernandez, Héctor; Pramparo, María C; Gayol, María F
2018-03-01
The objective of this study was to evaluate the antioxidant activity of rosemary essential oil fractions obtained by molecular distillation (MD) and investigate their effect on the oxidative stability of sunflower oil. MD fractions were prepared in a series of low-pressure stages where rosemary essential oil was the first feed. Subsequently, a distillate (D1) and residue (R1) were obtained and the residue fraction from the previous stage used as the feed for the next. The residue fractions had the largest capacity to capture free radicals, and the lowest peroxide values, conjugated dienes and conjugated trienes. The antioxidant activity of the fractions was due to oxygenated monoterpenes, specifically α-terpineol and cis-sabinene hydrate. Oxidative stability results showed the residues (R1 and R4) and butylated hydroxytoluene had greater antioxidant activity than either the distillate fractions or original rosemary essential oil. The residue fractions obtained by short path MD of rosemary essential oil could be used as a natural antioxidants by the food industry. Copyright © 2017. Published by Elsevier Ltd.
-
Synthetic lipophilic antioxidant BO-653 suppresses HCV replication.
Yasui, Fumihiko; Sudoh, Masayuki; Arai, Masaaki; Kohara, Michinori
2013-02-01
The influence of the intracellular redox state on the hepatitis C virus (HCV) life cycle is poorly understood. This study demonstrated the anti-HCV activity of 2,3-dihydro-5-hydroxy-2,2-dipentyl-4,6-di-tert-butylbenzofuran (BO-653), a synthetic lipophilic antioxidant, and examined whether BO-653's antioxidant activity is integral to its anti-HCV activity. The anti-HCV activity of BO-653 was investigated in HuH-7 cells bearing an HCV subgenomic replicon (FLR3-1 cells) and in HuH-7 cells infected persistently with HCV (RMT-tri cells). BO-653 inhibition of HCV replication was also compared with that of several hydrophilic and lipophilic antioxidants. BO-653 suppressed HCV replication in FLR3-1 and RMT-tri cells in a concentration-dependent manner. The lipophilic antioxidants had stronger anti-HCV activities than the hydrophilic antioxidants, and BO-653 displayed the strongest anti-HCV activity of all the antioxidants examined. Therefore, the anti-HCV activity of BO-653 was examined in chimeric mice harboring human hepatocytes infected with HCV. The combination treatment of BO-653 and polyethylene glycol-conjugated interferon-α (PEG-IFN) decreased serum HCV RNA titer more than that seen with PEG-IFN alone. These findings suggest that both the lipophilic property and the antioxidant activity of BO-653 play an important role in the inhibition of HCV replication. Copyright © 2012 Wiley Periodicals, Inc.
-
Directory of Open Access Journals (Sweden)
Alexander V. Vavaev
2012-02-01
Full Text Available The focus in antioxidant research is on enzyme derivative investigations. Extracellular superoxide dismutase (EC-SOD is of particular interest, as it demonstrates in vivo the protective action against development of atherosclerosis, hypertension, heart failure, diabetes mellitus. The reliable association of coronary artery disease with decreased level of heparin-released EC-SOD was established in clinical research. To create a base for and to develop antioxidant therapy, various SOD isozymes, catalase (CAT, methods of gene therapy, and combined applications of enzymes are used. Covalent bienzyme SOD-CHS-CAT conjugate (CHS, chondroitin sulphate showed high efficacy and safety as the drug candidate. There is an evident trend to use the components of glycocalyx and extracellular matrix for target delivery of medical substances. Development of new enzyme antioxidants for therapeutic application is closely connected with progress in medical biotechnology, pharmaceutical industry, and bioeconomy.
-
Ramel, A; Wagner, K; Elmadfa, I
2004-01-01
Objectives: To investigate noradrenaline concentrations, neutrophil counts, plasma antioxidants, and lipid oxidation products before and after acute resistance exercise. Methods: 17 male participants undertook a submaximal resistance exercise circuit (10 exercises; 75% of the one repetition maximum; mean (SD) exercise time, 18.6 (1.1) minutes). Blood samples were taken before and immediately after exercise and analysed for plasma antioxidants, noradrenaline, neutrophils, and lipid oxidation products. Wilcoxon's signed-rank test and Pearson's correlation coefficient were used for calculations. Results: Neutrophils, noradrenaline, fat soluble antioxidants, and lipid oxidation products increased after exercise. Noradrenaline concentrations were associated with higher antioxidant concentrations. Neutrophils were related to higher concentrations of conjugated dienes. Conclusions: Submaximal resistance exercise increases plasma antioxidants. This might reflect enhanced antioxidant defence in response to the oxidative stress of exercise, though this is not efficient for inhibiting lipid oxidation. The correlation between noradrenaline concentrations and plasma antioxidants suggests a modulating role of the stress hormone. Neutrophils are a possible source of oxidative stress after resistance exercise. PMID:15388566
-
Riboflavin Phototransformation on the Changes of Antioxidant Capacities in Phenolic Compounds.
Song, Juhee; Seol, Nam Gyu; Kim, Mi-Ja; Lee, JaeHwan
2016-08-01
Eight phenolic compounds including: p-coumaric acid, vanillic acid, caffeic acid, chlorogenic acid, trolox, quercetin, curcumin, and resveratrol were treated with riboflavin (RF) photosensitization and in vitro antioxidant capacities of the mixtures were determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2' azino bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), and ferric reducing antioxidant power (FRAP) assays. Mixtures containing p-coumaric acid and vanillic acid under RF photosensitization showed increases in ferric ion reducing ability and radical scavenging activity of DPPH, whereas mixtures of other compounds had decreases in both radical scavenging ability and ferric reducing antioxidant power. Hydroxycoumaric acid and conjugated hydroxycoumaric and coumaric acids were tentatively identified from RF photosensitized p-coumaric acid, whereas dimmers of vanillic acid were tentatively identified from RF photosensitized vanillic acid. RF photosensitization may be a useful method to enhance antioxidant properties like ferric ion reducing abilities of some selected phenolic compounds. © 2016 Institute of Food Technologists®
-
Antioxidant effects of an ozonized theobroma oil formulation on damaged-inflammatory rat skin
Energy Technology Data Exchange (ETDEWEB)
Sanchez, Y.; Diaz, M.F.; Hernandez, F.; Gila, D.; Ga, G.
2011-07-01
The aim of this study was to determine whether a cosmetic formulation elaborated with ozonized theobroma oil may exert beneficial effects in the restoring of the antioxidant activity on the skin of rats previously irradiated with ultraviolet light. 0.5 g of the formulation was applied on the skin of rats for five days. Superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) activity were determined in a homogenate of rat skin. Malondialdehyde (MDA), conjugated dienes (CD) and total hydroperoxide (THP) content were determined as biomarkers of oxidative stress. Using these parameters, antioxidant and oxidant activity, redox index and oxidative stress grade were determined. The total antioxidant activity was significantly increased while the redox index, total oxidant activity and oxidative stress grade decreased significantly in damaged rats treated with the formulation. These results show the antioxidant properties of the cosmetic formulation due to the stimulation of antioxidant enzymes such as SOD and GPx, preventing skin injury induced by ultraviolet irradiation. (Author).
-
Zbikowska, Halina Malgorzata; Szejk, Magdalena; Saluk, Joanna; Pawlaczyk-Graja, Izabela; Gancarz, Roman; Olejnik, Alicja Klaudia
2016-05-01
Polyphenolic-polysaccharide macromolecular, water-soluble glycoconjugates, isolated from the selected medicinal plants of Rosaceae/Asteraceae family: from leaves of Fragaria vesca L., Rubus plicatus Whe. et N. E., and from flowering parts of Sanguisorba officinalis L., and Erigeron canadensis L., were investigated for their ability to protect proteins and lipids of human plasma against γ-radiation-induced oxidative damage. Treatment of plasma with plant conjugates (6, 30, 150 μg/ml) prior exposure to 100 Gy radiation resulted in a significant inhibition of lipid peroxidation, evaluated by TBARS levels; conjugates isolated from E. canadensis and R. plicatus and a reference flavonoid quercetin showed similar high potential (approx. 70% inhibition, at 6 μg/ml). The conjugates prevented radiation-induced oxidation of protein thiols and significantly improved plasma total antioxidant capacity, estimated with Ellman's reagent and ABTS(.+) assay, respectively. The results demonstrate by the first time a significant radioprotective capability of the polyphenolic-polysaccharide conjugates isolated from E. canadensis, R. plicatus, S. officinalis and to the less extent from F. vesca. The abilities of these substances to inhibit radiation-induced lipid peroxidation and thiol oxidation in plasma seems to be mediated, but not limited to ROS scavenging activity. Copyright © 2016 Elsevier B.V. All rights reserved.
-
Curcumin-albumin conjugates as an effective anti-cancer agent with immunomodulatory properties.
Aravind, S R; Krishnan, Lissy K
2016-05-01
Curcumin (diferuloylmethane) is an active ingredient in turmeric (Curcuma longa) with anti-inflammatory, antioxidant, chemopreventive, chemosensitization, and radiosensitization properties. Conjugation of curcumin (Curc) to albumin (Alb) has been found to increase the aqueous solubility of the drug. The current study aimed to prove the safe use of the Curc-Alb conjugate in animals and to demonstrate that it retains drug action both in vitro and in vivo. Dalton's lymphoma ascites (DLA) cell viability was inhibited by the Curc-Alb conjugate in a dose dependent manner in vitro, as evidenced by the MTT assay. Administration of up to 11.4 mg of conjugated curcumin per kg body weight to healthy animals was non-toxic both in terms of lethality and weight loss. Histological analysis of vital organs (kidney, liver and spleen) also did not show toxic effects. Favorable immuno-modulatory activity was observed after continuous administration of sub-acute doses of the conjugate which caused increase in total leukocyte count, platelet count, and viable cell count in bone marrow, and enhanced proliferation of lymphocyte in vitro upon culture. In vivo studies in the DLA tumor model in mice demonstrated that conjugated drug induces tumor reduction and prevention. Significant tumor reduction was observed when the Curc-Alb conjugate was administered intraperitoneally in DLA-induced mice after 1 day (prevention therapy) and 7 days (reduction therapy) of tumor induction. There was significant reduction in both tumor volume and tumor cell numbers in the treated animals as well as a marked increase in their mean survival time and percent increase in life span. The effect was greater when the conjugate was administered soon after inducing the tumor as compared to when treatment was started after allowing tumor to grow for 7 days. Thus, the results of the present study suggest that curcumin albumin conjugate has immunomodulatory and tumor growth inhibition properties. The study postulates
-
Arokoyo, Dennis Seyi; Oyeyipo, Ibukun Peter; Du Plessis, Stefan Simon; Aboua, Yapo Guillaume
2018-01-01
Oxidative stress is frequently identified as a key element in the pathophysiology of many complications of diabetes mellitus, including reproductive complications. The antioxidant potential of medicinal plants have been suggested for therapeutic focus of diseases in recent reports. To investigate the effect of Basella alba (Ba) aqueous leave extract on diabetes-induced oxidative stress. Forty male Wistar rats (8-10 weeks) were randomly divided into four groups ( n = 10) and treated as follows; Control (C + Ns) and Diabetic (D + Ns) animals received oral normal saline 0.5 ml/100 g body weight daily, while Healthy Treatment (H + Ba) and Diabetic Treatment (D + Ba) rats were given Ba extract at an oral dose of 200 mg/kg body weight daily. Treatment was by gavage and lasted 4 weeks in all groups. Diabetes was induced in D + Ns and D + Ba rats by single intraperitoneal injection of streptozotocin (55 mg/kg) and fasting blood sugar (FBS) recorded weekly in all rats afterwards. Animals were euthanized at the end of the experiment and blood samples, pancreas, testes, and epididymis were preserved for analysis of oxidative stress biomarkers. Oral administration of aqueous leave extract of Ba significantly ( P antioxidant power, but lower serum concentration of conjugated dienes and thiobarbituric acid reactive substances in D + Ba compared to D + Ns rats ( P antioxidant effects in the gonads by enhancing antioxidant parameters in circulating blood, but not necessarily in the gonadal tissues. Oral treatment of diabetic rats with aqueous leave extract of Basella alba exerts antioxidant effects in the gonads by enhancing antioxidant parameters in circulating blood, but not necessarily in the gonadal tissues. Abbreviations Used: AP - Antioxidant parameters, Ba - Basella alba , CAT - Catalase, CDs - Conjugated dienes, DM - Diabetes mellitus, FBS - Fasting blood sugar, FRAP - Ferric reducing antioxidant power, GSH - reduced glutathione, Ns - Normal saline, ORAC - oxygen radical
-
Energy Technology Data Exchange (ETDEWEB)
Anwar, F.; Abdul Qayyum, H. M.; Hussein, A. I.; Iqbal, S.
2010-07-01
The antioxidant potential of 100% and 80% methanol extracts from the seeds of three barley varieties (Jou 83, Jou 87 and Haider 93) was assessed. The extract yields from barley seeds ranged from 3.23% (Haider 93,100% methanol) to 5.31% (Jou 83, 80% methanol). The total phenolic contents, DPPH radical scavenging activity (IC50 values) and inhibition of linoleic acid oxidation of barley seed extracts (BSE) were determined to be 88.1-145.7 mg/100g, 90.8-168.6 {mu}g/mL and 62.6-74.6%, respectively. The antioxidant effectiveness of BSE was also assessed by stabilizing sunflower oil (SFO) with BSE at a concentration of 600 ppm (oil weight basis). The stabilized (treated with extract) and the control (without extract addition) SFO samples were subjected to accelerated (oven heating at 60 degree centigrade for 30 days, 8 h heating cycle/day) storage. These were analyzed at regular intervals for the extent of oxidative changes according to the measurements of their contents of peroxide value, para-anisidine value, conjugated dienes and conjugated trienes. Generally, the 80% methanol extract of barely seeds demonstrated better antioxidant action than the 100% methanol extract. The antioxidant activity of BSE was also found to be considerably varied among the varieties tested. The present results suggest that antioxidant extracts from barely seeds might be used to protect vegetable oils from oxidation. (Author) 32 refs.
-
Purification of SUMO conjugating enzymes and kinetic analysis of substrate conjugation
Yunus, Ali A.; Lima, Christopher D.
2009-01-01
SUMO conjugation to protein substrates requires the concerted action of a dedicated E2 ubiquitin conjugation enzyme (Ubc9) and associated E3 ligases. Although Ubc9 can directly recognize and modify substrate lysine residues that occur within a consensus site for SUMO modification, E3 ligases can redirect specificity and enhance conjugation rates during SUMO conjugation in vitro and in vivo. In this chapter, we will describe methods utilized to purify SUMO conjugating enzymes and model substrates which can be used for analysis of SUMO conjugation in vitro. We will also describe methods to extract kinetic parameters during E3-dependent or E3-independent substrate conjugation. PMID:19107417
-
Characteristics and enhanced antioxidant activity of glycated Morchella esculenta protein isolate
Directory of Open Access Journals (Sweden)
Qiang ZHANG
Full Text Available Abstract Morchella esculenta (L Pers. is a highly valued edible and medicinal fungus that remains underutilized. For this study, the effects of glycation treatment on antioxidant activity and characteristics of the M. esculenta protein isolate (MPI were investigated via the Maillard reaction. Conjugation between MPI and xylose was proven via UV-vis, FT-IR, intrinsic fluorescence analysis, and SDS-PAGE. Amino acid analysis revealed involvement of lysine, arginine and tyrosine in MPI, forming a covalent cross-link with xylose. Differential scanning calorimetry (DSC results showed that glycated MPI (MPIG possesses a more favorable thermal stability compared to native MPI (MPIN, heated MPI (MPIH and an unheated mixture of MPI and xylose (MPI-XM. MPIG exhibited significantly enhanced antioxidant activity compared to MPIN, MPIH, and MPI-XM. These results indicate MPIG can serve as a promising novel source of nutraceutical and functional ingredients that exert antioxidant activity.
-
Waghela, Bhargav N.; Sharma, Anupama; Dhumale, Suhashini; Pandey, Shashibahl M.; Pathak, Chandramani
2015-01-01
Curcumin, an ingredient of turmeric, exhibits a variety of biological activities such as anti-inflammatory, anti-atherosclerotic, anti-proliferative, anti-oxidant, anti-cancer and anti-metastatic. It is a highly pleiotropic molecule that inhibits cell proliferation and induces apoptosis in cancer cells. Despite its imperative biological activities, chemical instability, photo-instability and poor bioavailability limits its utilization as an effective therapeutic agent. Therefore, enhancing the bioavailability of curcumin may improve its therapeutic index for clinical setting. In the present study, we have conjugated curcumin with a biodegradable polymer Poly (D, L-lactic-co-glycolic acid) and evaluated its apoptotic potential in human colon carcinoma cells (HCT 116). The results show that curcumin-PLGA conjugate efficiently inhibits cell proliferation and cell survival in human colon carcinoma cells as compared to native curcumin. Additionally, curcumin conjugated with PLGA shows improved cellular uptake and exhibits controlled release at physiological pH as compared to native curcumin. The curcumin-PLGA conjugate efficiently activates the cascade of caspases and promotes intrinsic apoptotic signaling. Thus, the results suggest that conjugation potentiates the sustainability, anti-proliferative and apoptotic activity of curcumin. This approach could be a promising strategy to improve the therapeutic index of cancer therapy. PMID:25692854
-
Gorbenko, M V; Popova, T N; Shul'gin, K K; Popov, S S
2013-01-01
Investigation of glutathione antioxidant system activity and diene conjugates content in rats liver and blood serum at the influence of melaksen and valdoxan under experimental hyperthyroidism (EG) has been revealed. It has been established that the activities of glutathione reductase (GR), glutathione peroxidase (GP) and glutathione transferase (GT), growing at pathological conditions, change to the side of control value at these substunces introduction. Reduced glutathione content (GSH) at melaxen and valdoxan action increased compared with values under the pathology, that, obviously, could be associated with a reduction of its spending on the detoxication of free radical oxidation (FRO) toxic products. Diene conjugates level in rats liver and blood serum, increasing at experimental hyperthyroidism conditions, under introduction of melatonin level correcting drugs, also approached to the control meaning. Results of the study indicate on positive effect of melaxen and valdoxan on free radical homeostasis, that appears to be accompanied by decrease of load on the glutathione antioxidant system in comparison with the pathology.
-
Directory of Open Access Journals (Sweden)
Sanna V
2014-10-01
, RSV, and FS, respectively. Nanoprototypes exhibited remarkable in vitro stability in various media, suggesting that NP surface coating with phytochemicals prevents aggregation in different simulated physiological conditions. The scavenging activities for DPPH and ABTS were highly correlated with EGCG, RSV, and FS content. Moreover, high correlation coefficients between the ABTS and DPPH values were found for the prepared nanosystems. EGCG-GNPs induce a dose-dependent reduction on SH-SY5Y-CFP-DEVD-YFP cell viability that is likely to involve the activation of the apoptotic pathways, similarly to free EGCG, as suggested by the processing of the CFP-DEVD-YFP reporter. Conclusion: These results prompted us to propose the ecofriendly synthesized EGCG-, RSV-, and FS-based nanogold conjugates as suitable carriers for bioactive polyphenols to be used for the treatment of disorders associated with oxidative stress, including neurodegenerative disorders, cardiovascular disease, and cancer. Keywords: gold nanoparticles, epigallocatechin-3-gallate, resveratrol, fisetin, antioxidant activity, SH-SY5Y-CFP-DEVD-YFP cells
-
Directory of Open Access Journals (Sweden)
Mustafa Atalay
2003-06-01
Full Text Available Regular physical exercise beneficially influences cardiac antioxidant defenses in normal rats. The aim of this study was to test whether endurance training can strengthen glutathione-dependent antioxidant defense mechanism and decrease lipid peroxidation in heart of the streptozotocin-induced diabetic rats. Redox status of glutathione in blood of diabetic rats in response to training and acute exercise was also examined. Eight weeks of treadmill training increased the endurance in streptozotocin-induced diabetic rats. It did not affect glutathione level in heart tissue at rest and also after exercise. On the other hand, endurance training decreased glutathione peroxidase activity in heart, while glutathione reductase and glutathione S-transferase activities were not affected either by acute exhaustive exercise or endurance training. Reduced and oxidized glutathione levels in blood were not affected by either training or acute exercise. Conjugated dienes levels in heart tissue were increased by acute exhaustive exercise and also 8 weeks treadmill training. Longer duration of exhaustion in trained group may have contributed to the increased conjugated dienes levels in heart after acute exercise. Our results suggest that endurance type exercise may make heart more susceptible to oxidative stress. Therefore it may be wise to combine aerobic exercise with insulin treatment to prevent its adverse effects on antioxidant defense in heart in patients with diabetes mellitus
-
Phenolic Content and Their Antioxidant Activity in Various Berries Cultivated in Romania
Directory of Open Access Journals (Sweden)
Zoriţa Diaconeasa
2015-05-01
Full Text Available Berry fruits are a rich source of phenolic compounds with health benefits. Phenolic compounds occur in berries mainly as a variety of conjugated forms, mostly with sugars. The aim of this work was to evaluate and compare the phenolic content and antioxidant potential in the most common fruits consumed in Romania: blueberry, blackberries, raspberry and cranberries. Folin-Ciocalteu method has been used in order to evaluate total phenolic content of analyzed berries. Antioxidant activity was determinate using ORAC assay which measures the decrease of AAPH-radical level by the scavenging action of the antioxidant substance. In addition, the vitamin C content and total tannins of the berries extracts were determined using spetophomotmetric methods. The phenolic contents and antioxidant potential of analyzed berries did not varied considerably. The highest amounts of TPC and the strongest antioxidant activities were found in blueberry and blackberries (678 GAE mg/100 g FW, 442 mg/100g FW respectively. Vitamin C content was found in higher concentration in raspberries 21.7 mg/100 g FW while the lower concentration was found in blackberry. All berries contain higher levels of bioactive compounds such as polyphenols or tannins which are responsible for their antioxidant potential and bring their nutritional value, being highly recommended for daily consumption.
-
Performance of Different Natural Antioxidant Compounds in Frying Oil
Directory of Open Access Journals (Sweden)
Buket Aydenız
2016-01-01
Full Text Available In this study, the natural green tea extract, purified lycopene, purified resveratrol and purified γ-oryzanol were added into peanut oil and their antioxidant performances were evaluated during frying. Moreover, the sensory properties of fried dough were evaluated to determine the consumption feasibility. All natural antioxidants led to significant increase in the stability of the oil samples. The ranges of measurements in the treatment groups were as follows: free acidity 0.1–2.9 g of oleic acid per 100 g of oil, conjugated dienes 0.01–0.40 g per 100 g of oil, total polar material 8.8–73.8 g per 100 g of oil, total phenolics 0.1–4.2 mg of gallic acid equivalents per 100 g of oil, and antioxidant capacity 0.5–11.0 mM of Trolox equivalents per 100 g of oil. The fatty acid and sterol compositions indicated that antioxidant supplementation could slow the oxidative degradation of unsaturated fatty acids and reduce trans-acid formation. Frying oil enriched with purified γ-oryzanol had higher sterol levels than the other enriched oil samples. The obtained quality of oil protection was in descending order: purified γ-oryzanol, green tea extract and purified lycopene.
-
International Nuclear Information System (INIS)
Trevisan, Rafael; Arl, Miriam; Sacchet, Cássia Lopes; Engel, Cristiano Severino; Danielli, Naissa Maria; Mello, Danielle Ferraz; Brocardo, Caroline; Maris, Angelica Francesca; Dafre, Alcir Luiz
2012-01-01
Disturbances in antioxidant defenses decrease cellular protection against oxidative stress and jeopardize cellular homeostasis. To knock down the antioxidant defenses of Pacific oyster Crassostrea gigas, animals were pre-treated with 1-chloro-2,4-dinitrobenzene (CDNB) and further challenged with pro-oxidant menadione (MEN). CDNB pre-treatment (10 μM for 18 h) was able to consume cellular thiols in gills, decreasing GSH (53%) and decrease protein thiols (25%). CDNB pre-treatment also disrupted glutathione reductase and thioredoxin reductase activity in the gills, but likewise strongly induced glutathione S-transferase activity (270% increase). Surprisingly, hemocyte viability was greatly affected 24 h after CDNB removal, indicating a possible vulnerability of the oyster immune system to electrophilic attack. New in vivo approaches were established, allowing the identification of higher rates of GSH–CDNB conjugate export to the seawater and enabling the measurement of the organic peroxide consumption rate. CDNB-induced impairment in antioxidant defenses decreased the peroxide removal rate from seawater. After showing that CDNB decreased gill antioxidant defenses and increased DNA damage in hemocytes, oysters were further challenged with 1 mM MEN over 24 h. MEN treatment did not affect thiol homeostasis in gills, while CDNB pre-treated animals recovered GSH and PSH to the control level after 24 h of depuration. Interestingly, MEN intensified GSH and PSH loss and mortality in CDNB-pre-treated animals, showing a clear synergistic effect. The superoxide-generating one-electron reduction of MEN was predominant in gills and may have contributed to MEN toxicity. These results support the idea that antioxidant-depleted animals are more susceptible to oxidative attack, which can compromise survival. Data also corroborate the idea that gills are an important detoxifying organ, able to dispose of organic peroxides, induce phase II enzymes, and efficiently export GSH�
-
Energy Technology Data Exchange (ETDEWEB)
Trevisan, Rafael; Arl, Miriam [Departamento de Bioquimica, Universidade Federal de Santa Catarina, 88040-900 Florianopolis, SC (Brazil); Sacchet, Cassia Lopes [Universidade do Oeste do Estado de Santa Catarina, 89600-000 Joacaba, SC (Brazil); Engel, Cristiano Severino; Danielli, Naissa Maria; Mello, Danielle Ferraz [Departamento de Bioquimica, Universidade Federal de Santa Catarina, 88040-900 Florianopolis, SC (Brazil); Brocardo, Caroline [Universidade do Oeste do Estado de Santa Catarina, 89600-000 Joacaba, SC (Brazil); Maris, Angelica Francesca [Departamento de Biologia Celular, Embriologia e Genetica, Universidade Federal de Santa Catarina, 88040-900 Florianopolis, SC (Brazil); Dafre, Alcir Luiz, E-mail: alcir@ccb.ufsc.br [Departamento de Bioquimica, Universidade Federal de Santa Catarina, 88040-900 Florianopolis, SC (Brazil)
2012-02-15
Disturbances in antioxidant defenses decrease cellular protection against oxidative stress and jeopardize cellular homeostasis. To knock down the antioxidant defenses of Pacific oyster Crassostrea gigas, animals were pre-treated with 1-chloro-2,4-dinitrobenzene (CDNB) and further challenged with pro-oxidant menadione (MEN). CDNB pre-treatment (10 {mu}M for 18 h) was able to consume cellular thiols in gills, decreasing GSH (53%) and decrease protein thiols (25%). CDNB pre-treatment also disrupted glutathione reductase and thioredoxin reductase activity in the gills, but likewise strongly induced glutathione S-transferase activity (270% increase). Surprisingly, hemocyte viability was greatly affected 24 h after CDNB removal, indicating a possible vulnerability of the oyster immune system to electrophilic attack. New in vivo approaches were established, allowing the identification of higher rates of GSH-CDNB conjugate export to the seawater and enabling the measurement of the organic peroxide consumption rate. CDNB-induced impairment in antioxidant defenses decreased the peroxide removal rate from seawater. After showing that CDNB decreased gill antioxidant defenses and increased DNA damage in hemocytes, oysters were further challenged with 1 mM MEN over 24 h. MEN treatment did not affect thiol homeostasis in gills, while CDNB pre-treated animals recovered GSH and PSH to the control level after 24 h of depuration. Interestingly, MEN intensified GSH and PSH loss and mortality in CDNB-pre-treated animals, showing a clear synergistic effect. The superoxide-generating one-electron reduction of MEN was predominant in gills and may have contributed to MEN toxicity. These results support the idea that antioxidant-depleted animals are more susceptible to oxidative attack, which can compromise survival. Data also corroborate the idea that gills are an important detoxifying organ, able to dispose of organic peroxides, induce phase II enzymes, and efficiently export GSH
-
Kim, Min Jun; Lee, Yonghyun; Jon, Sangyong; Lee, Dong Yun
2017-07-01
Transplanted islets suffer hypoxic stress, which leads to nonspecific inflammation. This is the major cause of islet graft failure during the early stage of intrahepatic islet transplantation. Although bilirubin has shown potent anti-oxidative and anti-inflammatory functions, its clinical applications have been limited due to its insolubility and short half-life. To overcome this problem, novel amphiphilic bilirubin nanoparticles are designed. Hydrophilic poly(ethylene glycol) (PEG) is conjugated to the hydrophobic bilirubin molecule. Then, the PEG-bilirubin conjugates form nanoparticles via self-assembly, i.e., so-called to BRNPs. BRNPs can protect islet cells not only from chemically induced oxidative stress by scavenging reactive oxygen species molecules, but also from activated macrophages by suppressing cytokine release. Importantly, in vivo experiments demonstrate that BRNP treatment can dramatically and significantly prolong islet graft survival compared to bilirubin treatment. In addition, immunohistochemical analysis shows BRNPs have potent anti-oxidative and anti-inflammatory capabilities. Collectively, novel BRNPs can be a new potent remedy for successful islet transplantation. Copyright © 2017 Elsevier Ltd. All rights reserved.
-
Directory of Open Access Journals (Sweden)
Joomin Lee
2017-11-01
Full Text Available In this study, we report a new multifunctional nanoparticle with antioxidative and antibacterial activities in vitro. ZnO@GA nanoparticles were fabricated by coordinated covalent bonding of the antioxidant gallic acid (GA on the surface of ZnO nanoparticles. This addition imparts both antioxidant activity and high affinity for the bacterial cell membrane. Antioxidative activities at various concentrations were evaluated using a 2,2′-azino-bis(ethylbenzthiazoline-6-sulfonic acid (ABTS radical scavenging method. Antibacterial activities were evaluated against Gram-positive bacteria (Staphylococcus aureus: S. aureus, including several strains of methicillin-resistant S. aureus (MRSA, and Gram-negative bacteria (Escherichia coli. The functionalized ZnO@GA nanoparticles showed good antioxidative activity (69.71%, and the bactericidal activity of these nanoparticles was also increased compared to that of non-functionalized ZnO nanoparticles, with particularly effective inhibition and high selectivity for MRSA strains. The results indicate that multifunctional ZnO nanoparticles conjugated to GA molecules via a simple surface modification process displaying both antioxidant and antibacterial activity, suggesting a possibility to use it as an antibacterial agent for removing MRSA.
-
Shao, Yafang; Hu, Zhanqiang; Yu, Yonghong; Mou, Renxiang; Zhu, Zhiwei; Beta, Trust
2018-01-15
Soluble-free, soluble-conjugated, insoluble-bound phenolics and antioxidant activity, flavonoid (TFC), proanthocyanidins (TPAC), anthocyanins and minerals of fifteen whole rice grains with different colors were investigated. Soluble-free protocatechuic and vanillic acids were only quantified in black rice, which had the most quantities. Non-pigmented rice had no detectable conjugated protocatechuic and 2,5-dihydroxybenzoic acids both of which were found in black and red rice, respectively. The main bound phenolic acids were ferulic and p-coumaric, as well as 2,5-dihydroxybenzoic in red rice and protocatechuic and vanillic acids in black rice. Soluble-conjugated phenolics, TFC, and anthocyanins were negatively correlated with L ∗ , b ∗ , C and H° values. TPAC was positively correlated with a ∗ (Pblack rice groups. Copyright © 2017 Elsevier Ltd. All rights reserved.
-
Perea-Domínguez, Xiomara Patricia; Espinosa-Alonso, Laura Gabriela; Hosseinian, Farah; HadiNezhad, Mehri; Valdez-Morales, Maribel; Medina-Godoy, Sergio
2017-03-01
Jatropha curcas seed shells are the by-product obtained during oil extraction process. Recently, its chemical composition has gained attention since its potential applications. The aim of this study was to identify phenolic compounds profile from a non-toxic J. curcas shell from Mexico, besides, evaluate J. curcas shell methanolic extract (JcSME) antioxidant activity. Free, conjugate and bound phenolics were fractionated and quantified (606.7, 193.32 and 909.59 μg/g shell, respectively) and 13 individual phenolic compounds were detected by HPLC. The radical-scavenging activity of JcSME was similar to Trolox and ascorbic acid by DPPH assay while by ABTS assay it was similar to BHT. Effective antioxidant capacity by ORAC was found (426.44 ± 53.39 μmol Trolox equivalents/g shell). The Mexican non-toxic J. curcas shell is rich in phenolic compounds with high antioxidant activity; hence, it could be considerate as a good source of natural antioxidants.
-
Lokhmatikov, Alexey V.; Voskoboynikova, Natalia; Cherepanov, Dmitry A.; Skulachev, Maxim V.; Steinhoff, Heinz-Jürgen; Skulachev, Vladimir P.; Mulkidjanian, Armen Y.
2016-01-01
Molecules of mitochondrial cardiolipin (CL) get selectively oxidized upon oxidative stress, which triggers the intrinsic apoptotic pathway. In a chemical model most closely resembling the mitochondrial membrane—liposomes of pure bovine heart CL—we compared ubiquinol-10, ubiquinol-6, and alpha-tocopherol, the most widespread naturally occurring antioxidants, with man-made, quinol-based amphiphilic antioxidants. Lipid peroxidation was induced by addition of an azo initiator in the absence and presence of diverse antioxidants, respectively. The kinetics of CL oxidation was monitored via formation of conjugated dienes at 234 nm. We found that natural ubiquinols and ubiquinol-based amphiphilic antioxidants were equally efficient in protecting CL liposomes from peroxidation; the chromanol-based antioxidants, including alpha-tocopherol, were 2-3 times less efficient. Amphiphilic antioxidants, but not natural ubiquinols and alpha-tocopherol, were able, additionally, to protect the CL bilayer from oxidation by acting from the water phase. We suggest that the previously reported therapeutic efficiency of mitochondrially targeted amphiphilic antioxidants is owing to their ability to protect those CL molecules that are inaccessible to natural hydrophobic antioxidants, being trapped within respiratory supercomplexes. The high susceptibility of such occluded CL molecules to oxidation may have prompted their recruitment as apoptotic signaling molecules by nature. PMID:27313834
-
Directory of Open Access Journals (Sweden)
Hsieh DS
2012-03-01
Full Text Available Dar-Shih Hsieh1,2, Hsiu-Chin Lu5, Cheng-Cheung Chen1,3, Chang-Jer Wu1,*, Ming-Kung Yeh4,* 1Department of Food Science, National Taiwan Ocean University, Keelung, Taiwan; 2Division of Urology, 3Department of Pathology, Tri-Service General Hospital, 4Institute of Preventive Medicine, National Defence Medical Center, Taipei, Taiwan; 5Department of Laboratory Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan*These authors contributed equally to this workAbstract: Nanogold particles are commonly used in nanomedicine. We generated physical nanogold (pNG conjugated with different ratios of epigallocatechin-3-gallate (EGCG and evaluated its physicochemical properties, antioxidant activity, and cytotoxicity in vitro as well as anticancer activity in vivo. Results showed that the EGCG-pNG conjugates were successfully prepared at ratios between 23:1 and 23:5, with the percentage of EGCG content increasing with the EGCG:pNG ratio from 23:1 (2.0% ± 0.02% to 23:5 (28% ± 0.3%. EGCG-pNG particles at ratios of 23:1 and 23:5 demonstrated significantly decreased size from 500 to 20 nm and decreasing zeta potentials of 21 mV to –22 mV, respectively. At a ratio of 23:2.5, the EGCG-pNG particles (27% EGCG, 50 nm in size, zeta potential of –8 mV showed longer EGCG activity half-life (110 days vs 5 hours, controlled release (2 hours vs 30 minutes, and higher antioxidant activity (four times, as well as inhibition of tumor cell growth, than controls. The present study indicated that EGCG-pNG possesses promising therapeutic potential, based on its strong free-radical scavenging and anticancer activities.Keywords: EGCG, nanoparticles, antioxidant, antitumor activity, nanogold
-
Hirunpanich, Vilasinee; Utaipat, Anocha; Morales, Noppawan Phumala; Bunyapraphatsara, Nuntavan; Sato, Hitoshi; Herunsalee, Angkana; Suthisisang, Chuthamanee
2005-03-01
The present study quantitatively investigated the antioxidant effects of the aqueous extracts from dried calyx of Hibiscus sabdariffa LINN. (roselle) in vitro using rat low-density lipoprotein (LDL). Formations of the conjugated dienes and thiobarbituric acid reactive substances (TBARs) were monitored as markers of the early and later stages of the oxidation of LDL, respectively. Thus, we demonstrated that the dried calyx extracts of roselle exhibits strong antioxidant activity in Cu(2+)-mediated oxidation of LDL (proselle inhibited TBARs-formation with greater potency than 100 microM of vitamin E. In conclusion, this study provides a quantitative insight into the potent antioxidant effect of roselle in vitro.
-
Directory of Open Access Journals (Sweden)
Mohamed Touaibia
2012-12-01
Full Text Available Caffeic acid phenethyl ester (CAPE is a bioactive component isolated from propolis. A series of CAPE analogues was synthesized and their antiradical/antioxidant effects analyzed. The effect of the presence of the double bond and of the conjugated system on the antioxidant effect is evaluated with the analogues obtained from 3-(3,4-dihydroxyphenyl propanoic acid. Those obtained from 2-(3,4-dihydroxyphenyl acetic acid and 3,4-dihydroxybenzoic acid allow the evaluation of the effect of the presence of two carbons between the carbonyl and aromatic system.
-
Antioxidant Activity of Hispidin Oligomers from Medicinal Fungi: A DFT Study
Directory of Open Access Journals (Sweden)
El Hassane Anouar
2014-03-01
Full Text Available Hispidin oligomers are styrylpyrone pigments isolated from the medicinal fungi Inonotus xeranticus and Phellinus linteus. They exhibit diverse biological activities and strong free radical scavenging activity. To rationalize the antioxidant activity of a series of four hispidin oligomers and determine the favored mechanism involved in free radical scavenging, DFT calculations were carried out at the B3P86/6-31+G (d, p level of theory in gas and solvent. The results showed that bond dissociation enthalpies of OH groups of hispidin oligomers (ArOH and spin density delocalization of related radicals (ArO• are the appropriate parameters to clarify the differences between the observed antioxidant activities for the four oligomers. The effect of the number of hydroxyl groups and presence of a catechol moiety conjugated to a double bond on the antioxidant activity were determined. Thermodynamic and kinetic studies showed that the PC-ET mechanism is the main mechanism involved in free radical scavenging. The spin density distribution over phenoxyl radicals allows a better understanding of the hispidin oligomers formation.
-
Directory of Open Access Journals (Sweden)
Xican Li
2018-03-01
Full Text Available Two 2-phenyl-benzofurans, moracin C {2-[3′,5′-dihydroxy-4′-(3-methlbut-2-enylphenyl]-6-hydroxybenzofuran} and its isomer iso-moracin C{2-[3′,5′-dihydroxy-4′-(3-methlbut-1-enylphenyl]-6-hydroxybenzofuran}, were comparatively studied using redox-related antioxidant assays and non-redox antioxidant assays. Moracin C always resulted in higher IC50 values than iso-moracin C in the redox-related antioxidant assays, including •O2−-inhibition, Cu2+-reducing power, DPPH•-inhibition, and ABTS+•-inhibition assays. In the non-redox antioxidant assay, moracin C and iso-moracin C underwent similar radical-adduct-formation (RAF, evidenced by the peaks at m/z 704 and m/z 618 in HPLC-MS spectra. In conclusion, both moracin C and iso-moracin C can act as 2-phenyl-benzofuran antioxidants; their antioxidant mechanisms may include redox-related ET and H+-transfer, and non-redox RAF. A double bond at the conjugation position can enhance the redox-related antioxidant potential, but hardly affects the RAF potential.
-
Bai, Feng; Diao, Jiajing; Wang, Ying; Sun, Shixin; Zhang, Hongmei; Liu, Yunyun; Wang, Yanqing; Cao, Jian
2017-08-16
Curcumin is a dominating active component of Curcuma longa and has been studied widely because of its prominent biological activities. The extremely low aqueous solubility, stability, and bioavailability of curcumin limit its application in the field of medicine. In this study, we developed pectin-curcumin (PEC-CCM) conjugates that could self-assemble water-soluble nanomicelles in aqueous solution. The structure of PEC-CCM conjugates was characterized by ultraviolet-visible spectra, fluorescence spectra, Fourier transform infrared spectroscopy, and 1 H nuclear magnetic resonance spectroscopy. The thermal property of PEC-CCM conjugates was investigated by thermogravimetric analysis. It was found that PEC-CCM conjugates had formed nanomicelles in aqueous medium via self-assembly. These nanomicelles were observed as small spheres or ellipsoids and aggregated with a size range of 70-190 nm by transmission electron microscopy analysis. In a solution of nanomicelles, the stability of curcumin was improved, and its antioxidant property was preserved. The anticancer activity of PEC-CCM conjugates was quantified by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay using a hepatic cancer cell line (HepG2), a breast cancer cell line (MCF-7), a cervical cancer cell line (HeLa), and a human normal kidney cell line (293A). It was found that the curcumin of PEC-CCM conjugates had a more significant inhibitory effect on cancer cells and was less cytotoxic to normal cells than free curcumin was. PEC-CCM conjugates have great potential for some food and pharmaceutical applications.
-
Ka, HyeJung; Yi, BoRa; Kim, Mi-Ja; Lee, JaeHwan
2016-05-01
The antioxidant properties of selected amino acids were tested using in vitro assays and oil-in-water (O/W) emulsions under riboflavin (RF) photosensitization. Headspace oxygen content, lipid hydroperoxides, and conjugated dienes were determined for the degree of oxidation. Riboflavin photosensitization was adapted as the oxidation driving force. In vitro assays showed that cysteine had the highest antioxidant properties followed by tryptophan and tyrosine. However, in O/W emulsions under RF photosensitization, tyrosine inhibited lipid oxidation whereas tryptophan acted as a prooxidant. Tryptophan accelerated the rates of oxidation in O/W emulsion without RF. The antioxidant properties of amino acids differed depending on the antioxidant determination methods, oxidation driving forces, and food matrices. © 2016 Institute of Food Technologists®
-
de la Rosa, Laura A; Alvarez-Parrilla, Emilio; Shahidi, Fereidoon
2011-01-12
The phenolic composition and antioxidant activity of pecan kernels and shells cultivated in three regions of the state of Chihuahua, Mexico, were analyzed. High concentrations of total extractable phenolics, flavonoids, and proanthocyanidins were found in kernels, and 5-20-fold higher concentrations were found in shells. Their concentrations were significantly affected by the growing region. Antioxidant activity was evaluated by ORAC, DPPH•, HO•, and ABTS•-- scavenging (TAC) methods. Antioxidant activity was strongly correlated with the concentrations of phenolic compounds. A strong correlation existed among the results obtained using these four methods. Five individual phenolic compounds were positively identified and quantified in kernels: ellagic, gallic, protocatechuic, and p-hydroxybenzoic acids and catechin. Only ellagic and gallic acids could be identified in shells. Seven phenolic compounds were tentatively identified in kernels by means of MS and UV spectral comparison, namely, protocatechuic aldehyde, (epi)gallocatechin, one gallic acid-glucose conjugate, three ellagic acid derivatives, and valoneic acid dilactone.
-
International Nuclear Information System (INIS)
Hadley, S.W.; Wilbur, D.S.
1990-01-01
The preparations and conjugations of 2,3,5,6-tetrafluorophenyl 5-[125I/131I]iodo-4-pentenoate (7a) and 2,3,5,6-tetrafluorophenyl 3,3-dimethyl-5-[125I/131I]iodo-4-pentenoate (7b) to monoclonal antibodies are reported. Reagents 7a and 7b were prepared in high radiochemical yield by iododestannylation of their corresponding 5-tri-n-butylstannyl precursors. Radioiodinated antibody conjugates were prepared by reaction of 7a or 7b with the protein at basic pH. Evaluation of these conjugates by several in vitro procedures demonstrated that the radiolabel was attached to the antibody in a stable manner and that the conjugates maintained immunoreactivity. Comparative dual-isotope biodistribution studies of a monoclonal antibody Fab fragment conjugate of 7a and 7b with the same Fab fragment labeled with N-succinimidyl p-[131I]iodobenzoate (PIB, p-iodobenzoate, 2) or directly radioiodinated have been carried out in tumor-bearing nude mice. Coinjection of the Fab conjugate of 7a with the Fab conjugate of 2 demonstrated that the biodistributions were similar in most organs, except the neck tissue (thyroid-containing) and the stomach, which contained substantially increased levels of the 7a label. Coinjection of the Fab conjugate of 7a with the Fab fragment radioiodinated by using the chloramine-T method demonstrated that the biodistributions were remarkably similar, suggesting roughly equivalent in vivo deiodination of these labeled antibody fragments. Coinjection of the Fab conjugate of 7a with the Fab conjugate of 7b indicated that there was ∼ a 2-fold reduction in the amount of in vivo deiodination of the 7b conjugate as compared to the 7a conjugate
-
Directory of Open Access Journals (Sweden)
Monchai Dejsungkranont
2017-10-01
Full Text Available The functionality and activity of proteins can be modified by supercritical fluid CO2 (SCFCO2. The objectives of this study were to investigate the possibility of enhanced antioxidant activity of C-phycocyanin (C-PC proteins from light-harvested Spirulina maxima powder using the SCFCO2 pretreatment and to optimize the SCFCO2 pretreatment conditions enhancing the antioxidant activity of C-PC. The Taguchi method was used to determine the optimum conditions for the SCFCO2 pretreatment. The experimental factors were the pretreatment temperature, pressure, pretreatment mode (static, dynamic and conjugated and duration. The optimal conditions of SCFCO2 pretreatment were: 60 °C, 24.13 MPa and 60 min in static batch mode. Using these pretreatment conditions, the maximum antioxidant activity of C-PC from the treated residual biomass was 410.1 μmole trolox/mg, which was 1.7-fold higher than the untreated biomass (control. The factor that most affected the antioxidant activity of C-PC was temperature (59%. A high pretreatment temperature could damage C-PC, but promoted antioxidant activity. Of note is that this work was the first to explore SCFCO2 treatment enhancing the antioxidant activity of C-PC in Spirulina sp. powder. Keywords: Antioxidant activity, C-phycocyanin, Spirulina sp., Supercritical fluid carbon dioxide pretreatment, Taguchi method
-
Evaluation of the antioxidant activity of rice bran extracts using different antioxidant assays
Directory of Open Access Journals (Sweden)
Rehman Bajwa, Jawad -ur-
2006-09-01
Full Text Available In the present work the antioxidant activity of different solvent (100% methanol, 80% methanol, 100% acetone, 80% acetone extracts of rice bran was evaluated following different antioxidant assays and using sunflower oil as oxidation substrate. The rice bran extracts were evaluated from the estimate of % inhibition of peroxidation in linoleic acid system, total phenolics content (TPC and loss of β-carotene in a linoleic acid system. Additionally, crude concentrated rice bran extracts were added into the sunflower oil samples and stored under ambient conditions. The extent of oxidative deterioration was followed by the measurement of peroxide-, p-anisidine-, conjugated diene-, and triene- values. The general order of antioxidant efficacy of rice bran extracts as determined by various antioxidant assays was 80% methanolic extract > 100% methanolic extract > 80% acetone extract > 100% acetone extract. The results of the present comprehensive analysis demonstrate that rice bran extracts of the Super Kernel variety indigenous to Pakistan are a viable source of natural antioxidants and might be exploited for functional foods and nutraceutical applications.Se evalúa la actividad antioxidante diferentes extractos (100% metanol, 80% metanol, 100% acetona and 80% acetona de salvado de arroz -var. Super Kernel- mediante diferentes ensayos y utilizando aceite de girasol como substrato. Los ensayos utilizados fueron la estimación del % de inhibición de la peroxidación en sistemas con ácido linoleico, el contenido total en compuestos fenólicos y la pérdida de β-caroteno en sistemas con ácido linoleico. Adicionalmente, los concentrados de extractos de salvado de arroz se añadieron a aceite de girasol y las muestras se almacenaron a temperatura ambiente. La extensión de la oxidación se evaluó mediante el índice de peróxidos, el índice de p-anisidina, así como la formación de dienos y trienos conjugados. El orden de la eficacia antioxidante
-
Jadhav, Swati B; Singhal, Rekha S
2013-10-15
Two enzymes, α-amylase and glucoamylase have been individually and co-conjugated to pectin by covalent binding. Both the enzyme systems showed better thermal and pH stability over the free enzyme system with the complete retention of original activities. Mixture of individually conjugated enzymes showed lower inactivation rate constant with longer half life than the co-conjugated enzyme system. Individually conjugated enzymes showed an increase of 56.48 kJ/mole and 38.22 kJ/mole in activation energy for denaturation than the free enzymes and co-conjugated enzymes, respectively. Km as well as Vmax of individually and co-conjugated enzymes was found to be higher than the free enzymes. SDS-polyacrylamide gel electrophoresis confirmed the formation of conjugate and co-conjugate as evident by increased molecular weight. Both the enzyme systems were used for starch hydrolysis where individually conjugated enzymes showed highest release of glucose at 60 °C and pH 5.0 as compared to free and co-conjugated enzyme. Copyright © 2013 Elsevier Ltd. All rights reserved.
-
Directory of Open Access Journals (Sweden)
Sedighe Asgari
2010-12-01
Full Text Available Abstract INTRODUCTION: Myocardial ischemia, cerebral ischemia and myocardial infarction are the most important complications of hypertension and atherosclerotic disease in developing countries. Angiotensin converting enzyme (ACE inhibitors are among the drugs used to treat hypertension and heart failure. Captopril is an ACE-inhibitor which also has antioxidant properties. This study was conducted to assess the antioxidant effects of Iranian Captopril on malondialdehyde (MDA, conjugated dienes (CD and serum antioxidant capacity before and after treatment. methods: This interventional prospective single-blind study was conducted on 34 mildly hypertensive individuals and 34 patients with stage I and II heart failure. MDA, CD and serum antioxidant capacity were measured in all samples. The patients were then given 50 mg Captopril tablets 2-3 times daily. The measurements were repeated 1.5 months later. results: Comparison of mean MDA, CD and serum antioxidant capacity in hypertensive patients and patients with heart failure before and after drug administration revealed no significant difference in any of the parameters studied. Discussion: Existing evidence is suggestive of the strong antioxidative properties of Captopril in vitro, although these effects have not been borne out by some studies. In the present study, comparison of MDA, CD and serum antioxidants before and after the period of treatment with Iranian Captopril did not reveal any statistically significant difference.Keywords • Antioxidant • ACE inhibitor • High blood pressure • Heart failure • Clinical trial
-
role of conjugated linoleic acid in the prevention of radiation hazard in male rats
International Nuclear Information System (INIS)
Hussien, E.M.; Osman, N.N.; Haggag, A.M.
2009-01-01
the objective of the present study was to examine the effect of conjugated linoleic acid (CLA) as a natural product in minimizing the radiation hazards. male rats were assigned to six groups each of 7 animals throughout six weeks, fed 1% CLA (wt/wt)added to commercial diet in the form of milk powder 182 g/kg diet. rats exposed to 6 Gy whole body gamma irradiation showed significant increase in total cholesterol (TC), low density lipoprotein cholesterol (LDL-C).triglycerides (TG), atherosclerosis index, total lipid (TL), phospholipids (ph-lipids), malondialdehyde (MDA), urea,creatinine, uric acid, calcium (Ca) and phosphorous levels associated with decrease in high density lipoprotein-cholesterol (HDL-C), activity of reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), total antioxidant status, body weight, testes weight and testosterone both irradiated and non-irradiated milk powder administrated to irradiated rat groups minimized the radiation damage in the assayed parameters indicating its beneficial role as a promising antioxidant in scavenging free radicals and reactive oxygen species
-
Resveratrol-loaded Nanoparticles Induce Antioxidant Activity against Oxidative Stress
Directory of Open Access Journals (Sweden)
Jae-Hwan Kim
2016-02-01
Full Text Available Resveratrol acts as a free radical scavenger and a potent antioxidant in the inhibition of numerous reactive oxygen species (ROS. The function of resveratrol and resveratrol-loaded nanoparticles in protecting human lung cancer cells (A549 against hydrogen peroxide was investigated in this study. The 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS assay was performed to evaluate the antioxidant properties. Resveratrol had substantially high antioxidant capacity (trolox equivalent antioxidant capacity value compared to trolox and vitamin E since the concentration of resveratrol was more than 50 μM. Nanoparticles prepared from β-lactoglobulin (β-lg were successfully developed. The β-lg nanoparticle showed 60 to 146 nm diameter in size with negatively charged surface. Non-cytotoxicity was observed in Caco-2 cells treated with β-lg nanoparticles. Fluorescein isothiocynate-conjugated β-lg nanoparticles were identified into the cell membrane of Caco-2 cells, indicating that nanoparticles can be used as a delivery system. Hydrogen peroxide caused accumulation of ROS in a dose- and time-dependent manner. Resveratrol-loaded nanoparticles restored H2O2-induced ROS levels by induction of cellular uptake of resveratrol in A549 cells. Furthermore, resveratrol activated nuclear factor erythroid 2-related factor 2-Kelch ECH associating protein 1 (Nrf2-Keap1 signaling in A549 cells, thereby accumulation of Nrf2 abundance, as demonstrated by western blotting approach. Overall, these results may have implications for improvement of oxidative stress in treatment with nanoparticles as a biodegradable and non-toxic delivery carrier of bioactive compounds.
-
Fernandes, Carlos; Pinto, Miguel; Martins, Cláudia; Gomes, Maria João; Sarmento, Bruno; Oliveira, Paulo J; Remião, Fernando; Borges, Fernanda
2018-05-16
The uptake and transport of dietary antioxidants remains the most important setback for their application in therapy. To overcome the limitations, a PEGylated-based platform was developed to improve the delivery properties of two dietary hydroxycinnamic (HCA) antioxidants-caffeic and ferulic acids. The antioxidant properties of the new polymer-antioxidant conjugates (PEGAntiOxs), prepared by linking poly(ethylene glycol) (PEG) to the cinnamic acids by a one-step Knovenagel condensation reaction, were evaluated. PEGAntiOxs present a higher lipophilicity than the parent compounds (caffeic and ferulic acids) and similar, or higher, antioxidant properties. PEGAntiOxs were not cytotoxic at the tested concentrations in SH-SY5Y, Caco-2, and hCMEC/D3 cells. By contrast, cytotoxic effects in hCMEC/D3 and SH-SY5Y cells were observed, at 50 and 100 μM, for caffeic and ferulic acids. PEGAntiOxs operate as antioxidants against several oxidative stress-cellular inducers in a neuronal cell-based model, and were able to inhibit glycoprotein-P in Caco-2 cells. PEGAntiOxs can cross hCMEC/D3 monolayer cells, a model of the blood-brain barrier (BBB) endothelial membrane. In summary, PEGAntiOxs are valid antioxidant prototypes that can uphold the antioxidant properties of HCAs, reduce their cytotoxicity, and improve their BBB permeability. PEGAntiOxs can be used in the near future as drug candidates to prevent or slow oxidative stress associated with neurodegenerative diseases.
-
Bacteriophytochromes control conjugation in Agrobacterium fabrum.
Bai, Yingnan; Rottwinkel, Gregor; Feng, Juan; Liu, Yiyao; Lamparter, Tilman
2016-08-01
Bacterial conjugation, the transfer of single stranded plasmid DNA from donor to recipient cell, is mediated through the type IV secretion system. We performed conjugation assays using a transmissible artificial plasmid as reporter. With this assay, conjugation in Agrobacterium fabrum was modulated by the phytochromes Agp1 and Agp2, photoreceptors that are most sensitive in the red region of visible light. In conjugation studies with wild-type donor cells carrying a pBIN-GUSINT plasmid as reporter that lacked the Ti (tumor inducing) plasmid, no conjugation was observed. When either agp1(-) or agp2(-) knockout donor strains were used, plasmid DNA was delivered to the recipient, indicating that both phytochromes suppress conjugation in the wild type donor. In the recipient strains, the loss of Agp1 or Agp2 led to diminished conjugation. When wild type cells with Ti plasmid and pBIN-GUS reporter plasmid were used as donor, a high rate of conjugation was observed. The DNA transfer was down regulated by red or far-red light by a factor of 3.5. With agp1(-) or agp2(-) knockout donor cells, conjugation in the dark was about 10 times lower than with the wild type donor, and with the double knockout donor no conjugation was observed. These results imply that the phytochrome system has evolved to inhibit conjugation in the light. The decrease of conjugation under different temperature correlated with the decrease of phytochrome autophosphorylation. Copyright © 2015 Elsevier B.V. All rights reserved.
-
Polymers for Protein Conjugation
Directory of Open Access Journals (Sweden)
Gianfranco Pasut
2014-01-01
Full Text Available Polyethylene glycol (PEG at the moment is considered the leading polymer for protein conjugation in view of its unique properties, as well as to its low toxicity in humans, qualities which have been confirmed by its extensive use in clinical practice. Other polymers that are safe, biodegradable and custom-designed have, nevertheless, also been investigated as potential candidates for protein conjugation. This review will focus on natural polymers and synthetic linear polymers that have been used for protein delivery and the results associated with their use. Genetic fusion approaches for the preparation of protein-polypeptide conjugates will be also reviewed and compared with the best known chemical conjugation ones.
-
International Nuclear Information System (INIS)
Azab, Kh. SH.
2002-01-01
Eugenol, a volatile phenolic phyto chemical, is a major constituent of clove oil. The present study was carried out to evaluate the antioxidant effect of eugenol on certain lipid metabolites and variations in the antioxidant status. In vitro study (oxidative susceptibility of lipoprotein) revealed that eugenol elongates the lag phase for the induction of conjugated diene and decreased the rate of lipid peroxidation (production of thiobarbituric reactive substances; TBARS) during the propagation phase. In vivo study on rats revealed a significant increase in plasma total antioxidant status after eugenol regime. Furthermore, eugenol water emulsion delivered to rats by garage in a concentration of 1 g/kg body weight for 15 days before and during exposure to fractionated whole body gamma radiation (1.5 Gy every other day) up to a total dose of 7.5 Gy showed that, administration of eugenol reduces significantly the concentration of plasma TBARS and minimize the decrease in plasma antioxidants. Amelioration in the concentration of reduced glutathione (GSH) in blood and liver and the activities of cytosolic glutathione-S-transferase (GST) in the liver were also observed. Furthermore, the changes in the concentrations of total cholesterol, triglycerides, LDL-cholesterol and HDL-cholesterol were less pronounced. It could be postulated that by minimizing the decrease in antioxidant status, eugenol could prevents the radiation induce alterations in lipid metabolism
-
Pan, Li-Long; Wang, Jie; Jia, Yao-Ling; Zheng, Hong-Ming; Wang, Yang; Zhu, Yi-Zhun
2014-01-01
We have previously reported that the danshensu-cysteine conjugate N-((R)-3-benzylthio-1-methoxy-1-oxo-2-propanyl)-2-acetoxy-3-(3,4-diacetoxyphenyl) propanamide (DSC) is a potent anti-oxidative and anti-apoptotic agent. Herein, we further design and asymmetrically synthesize two diastereoisomers of DSC and explore their potential bioactivities. Our results show that DSC and its two diastereoisomers exert similar protective effects in hydrogen peroxide (H2O2)-induced cellular injury in SH-SY5Y cells, as evidenced by the increase of cell viability, superoxide dismutase (SOD), and reduced glutathione (GSH) activity, and glutathione peroxidase (GPx) expression, and the decrease of cellular morphological changes and nuclear condensation, lactate dehydrogenase (LDH) release, and malondialdehyde (MDA) production. In H2O2-stimulated human umbilical vein endothelial cells (HUVEC), DSC concentration-dependently attenuates H2O2-induced cell death, LDH release, mitochondrial membrane potential collapse, and modulates the expression of apoptosis-related proteins (Bcl-2, Bax, caspase-3, and caspase-9). Our results provide strong evidence that DSC and its two diastereoisomers have similar anti-oxidative activity and that DSC exerts significant vascular-protective effects, at least in part, through inhibition of apoptosis and modulation of endogenous antioxidant enzymes. PMID:25551606
-
Serum Antioxidative Enzymes Levels and Oxidative Stress Products in Age-Related Cataract Patients
Directory of Open Access Journals (Sweden)
Dong Chang
2013-01-01
Full Text Available Purpose. To investigate the activity of antioxidative enzymes and the products of oxidative stress in patients with age-related cataracts and compare the findings with those in healthy control subjects. Method. Sixty patients with age-related cataract and sixty healthy controls of matched age and gender were included in this study. Serum samples were obtained to detect the antioxidative enzymes of superoxide dismutase (SOD, catalase (CAT, and glutathione peroxidase (GSH-Px, and oxidation degradation products of malondialdehyde (MDA, 4-hydroxynonenal (4-HNE, conjugated diene (CD, advanced oxidation protein products (AOPP, protein carbonyl (PC, and 8-hydroxydeoxyguanosine (8-OHdG. Results. Serum SOD, GSH-Px, and CAT activities in cataract group were significantly decreased as compared to the control subjects (P<0.05. The levels of MDA, 4-HNE, and CD in cataract patients were significantly higher than those in the control subjects (P<0.05, P<0.01. Cataract patients had higher levels of 8-OHdG, AOPP, and PC with respect to the comparative group of normal subjects (P<0.01. And there was no statistical significance in concentration of antioxidative enzymes and oxidative stress products in patients with different subtype cataract. Conclusions. Oxidative stress is an important risk factor in the development of age-related cataract, and augmentation of the antioxidant defence systems may be of benefit to prevent or delay cataractogenesis.
-
Fisher, Robert A
1983-01-01
This book appears at a time of intense activity in optical phase conjugation. We chose not to await the maturation of the field, but instead to provide this material in time to be useful in its development. We have tried very hard to elucidate and interrelate the various nonlinear phenomena which can be used for optical phase conjugation.
-
Qualidade conjugal: mapeando conceitos
Directory of Open Access Journals (Sweden)
Clarisse Mosmann
2006-12-01
Full Text Available Apesar da ampla utilização do conceito de qualidade conjugal, identifica-se falta de clareza conceitual acerca das variáveis que o compõem. Esse artigo apresenta revisão da literatura na área com o objetivo de mapear o conceito de qualidade conjugal. Foram analisadas sete principais teorias sobre o tema: Troca Social, Comportamental, Apego, Teoria da Crise, Interacionismo Simbólico. Pelos postulados propostos nas diferentes teorias, podem-se identificar três grupos de variáveis fundamentais na definição da qualidade conjugal: recursos pessoais dos cônjuges, contexto de inserção do casal e processos adaptativos. Neste sentido, a qualidade conjugal é resultado do processo dinâmico e interativo do casal, razão deste caráter multidimensional.
-
Directory of Open Access Journals (Sweden)
Julio Zukerman-Schpector
2013-06-01
Full Text Available The analysis of the IR carbonyl bands of some 3-(4′-substituted phenylsulfanyl-1-methyl-2-piperidones 1–6 bearing substituents: NO2 (compound 1, Br (compound 2, Cl (compound 3, H (compound 4 Me (compound 5 and OMe (compound 6 supported by B3LYP/6-31+G(d,p and PCM calculations along with NBO analysis (for compound 4 and X-ray diffraction (for 2 indicated the existence of two stable conformations, i.e., axial (ax and equatorial (eq, the former corresponding to the most stable and the least polar one in the gas phase calculations. The sum of the energy contributions of the orbital interactions (NBO analysis and the electrostatic interactions correlate well with the populations and the νCO frequencies of the ax and eq conformers found in the gas phase. Unusually, in solution of the non-polar solvents n-C6H14 and CCl4, the more intense higher IR carbonyl frequency can be ascribed to the ax conformer, while the less intense lower IR doublet component to the eq one. The same νCO frequency trend also holds in polar solvents, that is νCO (eq< νCO (ax. However, a reversal of the ax/eq intensity ratio occurs going from non-polar to polar solvents, with the ax conformer component that progressively decreases with respect to the eq one in CHCl3 and CH2Cl2, and is no longer detectable in the most polar solvent CH3CN. The PCM method applied to compound 4 supports these findings. In fact, it predicts the progressive increase of the eq/ax population ratio as the relative permittivity of the solvent increases. Moreover, it indicates that the computed νCO frequencies of the ax and eq conformers do not change in the non–polar solvents n-C6H14 and CCl4, while the νCO frequencies of the eq conformer become progressively lower than that of the ax one going from CHCl3 to CH2Cl2 and to CH3CN, in agreement with the experimental IR values. The analysis of the geometries of the ax and eq conformers shows that the carbonyl oxygen atom of the eq conformer is free
-
ANTIOXIDANT EFFECTS OF L-SERINE AGAINST FATTY STREAK FORMATION IN HYPERCHOLESTEROLEMIC ANIMALS
Directory of Open Access Journals (Sweden)
Ahmad Movahedian
2010-12-01
Full Text Available Abstract INTRODUCTION: Peroxidation of blood lipoproteins is regarded as a key event in the development of atherosclerosis. Evidence suggests that oxidative modification of amino acids in low-density lipoprotein (LDL particles leads to its convert into an atherogenic form, which is taken up by macrophages. Therefore the reduction of oxidative modification of lipoproteins by increasing plasma antioxidant capacity may prevent cardiovascular disease. methods: In this study, the antioxidant and anti-fatty streak effects of L-serine were investigated in hypercholesterolemic rabbits. Rabbits were randomly divided into three groups which were fed high-cholesterol diet (hypercholesterolemic control group, high-cholesterol + L-serine diet (treatment group, and normal diet (control for twelve weeks and then blood samples were obtained to measure plasma cholesterol, triglyceride (TG, high-density lipoprotein (HDL, low-density lipoprotein (LDL, antioxidant capacity (AC, malondialdehyde (MDA, and conjugated dienes (CDS. Right and left coronary arteries were also obtained for histological evaluation. results: No significant difference was observed in plasma cholesterol, TG, HDL, LDL and CDS levels between treatment and hypercholesterolemic control groups (P>0.05. The levels of plasma MDA and AC were 0.29 µM and 56%, respectively in the treatment group which showed a significant change in comparison with hypercholesterolemic control groups (P<0.05. The mean size of produced fatty streak also showed significant reduction in the treatment group compared to the hypercholesterolemic group (P<0.05. CONCLUSIONS: The results showed that L-serine has antioxidant and anti-fatty streak effects without any influence on plasma lipid levels in hypercholesterolemic rabbits. Keywords: Atherosclerosis, cholesterol, L-serine, antioxidant, lipids, fatty streak.
-
Cross-Conjugated n-Dopable Aromatic Polyketone
Voortman, Thomas P.; Bartesaghi, Davide; Koster, L. Jan Anton; Chiechi, Ryan C.
2015-01-01
This paper describes the synthesis and characterization of a high molecular weight cross-conjugated polyketone synthesized via scalable Friedel Crafts chemistry. Cross-conjugated polyketones are precursors to conjugated polyions; they become orders of magnitude more conductive after a two-electron
-
Association of antioxidant nutraceuticals and acetaminophen (paracetamol: Friend or foe?
Directory of Open Access Journals (Sweden)
Mohamed Abdel-Daim
2018-04-01
Full Text Available Acetaminophen (paracetamol or APAP is an analgesic and antipyretic drug that can induce oxidative stress-mediated hepatotoxicity at high doses. Several studies reported that antioxidant nutraceuticals, in particular phenolic phytochemicals from dietary food, spices, herbs and algae have hepatoprotective effects. Others, however, suggested that they may negatively impact the metabolism, efficacy and toxicity of APAP. The aim of this review is to discuss the pros and cons of the association of antioxidant nutraceuticals and APAP by reviewing the in vivo evidence, with particular reference to APAP pharmacokinetics and hepatotoxicity. Results from the murine models of APAP-induced hepatotoxicity showed amelioration of liver damage with nutraceuticals coadministration, as well as reductions in tissue markers of oxidative stress, and serum levels of hepatic enzymes, bilirubin, cholesterol, triglycerides and inflammatory cytokines. On the other hand, both increased and decreased APAP plasma levels have been reported, depending on the nutraceutical type and route of administration. For example, studies showed that repeated administration of flavonoids causes down-regulation of cytochrome P450 enzymes and up-regulation of uridine diphosphate glucuronosyltransferases (UGT. Moreover, nutraceuticals can alter the levels of APAP metabolites, such as mercapturate glucuronide, sulfate and cysteine conjugates. Overall, the reviewed in vivo studies indicate that interactions between APAP and nutraceuticals or plant foods exist. However, the majority of data come from animal models with doses of phytochemicals far from dietary ones. Human studies should investigate gene-diet interactions, as well as ethnic variability in order to clarify the pros and cons of co-administering antioxidant nutraceuticals and APAP. Keywords: Acetaminophen, Antioxidants, Food-drug interaction, Nutraceuticals, Paracetamol
-
Phytochemicals and antioxidant capacity in four Italian traditional maize (Zea mays L.) varieties.
Capocchi, Antonella; Bottega, Stefania; Spanò, Carmelina; Fontanini, Debora
2017-08-01
Flours of four pigmented (from orange to red and dark red) local Italian corns, studied for their soluble, soluble conjugate, and insoluble-bound phenols and flavonoids, showed a prevalence of the insoluble-bound fraction (70-80%). Correlations were found between the flours antioxidant capacity, measured with CUPRAC, FRAP, and DPPH methods, and soluble phenols and flavonoids content. A correlation was also found between ascorbic acid content and flours antioxidant power. Anthocyanins were present in small amounts in the red/dark red seeds; however, acid-alcohol assays and spectral analyses of pericarp extracts indicated the presence of red-brick phlobaphenes in these varieties. Spectrophotometrically quantified total carotenoids were significantly higher in one of the local varieties (Nano); RP-HPLC analyses indicated that the local varieties contained significantly higher amounts of zeaxanthin and β-carotene, and lower amounts of lutein, than a commercial line. Among local varieties, Nano expressed the highest levels of zeaxanthin, β-carotene, and β-cryptoxanthin.
-
Ferulic acid modification enhances the anti-oxidation activity of natural Hb in vitro.
Qi, Donglai; Li, Qian; Chen, Chen; Wang, Xiang
2018-03-13
During the development of artificial red blood cell (RBC) substitutes, oxidation side reaction is one of the major factors that hinder the application of haemoglobin (Hb)-based oxygen carriers (HBOCs). In order to avoid oxidation toxicity, we designed and prepared natural Hb conjugated with ferulic acid (FA) via simple chemical modification. In addition, the thiol groups on Hb surface were increased via the reaction of Hb with 2-iminothiolane (2-IT) and then modified with FA for the study of anti-oxidant ability. It was showed that Hb modified with FA (FA-Hb) had similar oxygen-binding capacity to natural Hb. Moreover, the anti-oxidant ability of FA-Hb in vitro in different systems was superior to natural Hb and in proportion to the degree of modification of FA. The results indicate that FA-Hb might have the potential to serve as a novel oxygen carrier with the capacity to reduce oxidative side reaction.
-
Block-conjugate-gradient method
International Nuclear Information System (INIS)
McCarthy, J.F.
1989-01-01
It is shown that by using the block-conjugate-gradient method several, say s, columns of the inverse Kogut-Susskind fermion matrix can be found simultaneously, in less time than it would take to run the standard conjugate-gradient algorithm s times. The method improves in efficiency relative to the standard conjugate-gradient algorithm as the fermion mass is decreased and as the value of the coupling is pushed to its limit before the finite-size effects become important. Thus it is potentially useful for measuring propagators in large lattice-gauge-theory calculations of the particle spectrum
-
Jiménez-Aspee, Felipe; Thomas-Valdés, Samanta; Schulz, Ayla; Ladio, Ana; Theoduloz, Cristina; Schmeda-Hirschmann, Guillermo
2016-07-01
The Patagonian currant Ribes magellanicum is highly valued due to its pleasant flavor and sweet taste. The aim of this study was to characterize its constituents and to assess their antioxidant and cytoprotective properties. For the fruit phenolic-enriched extract (PEE), total phenolics (TP), total flavonoids (TF), and antioxidant activity (DPPH, Ferric reducing antioxidant power (FRAP), and Trolox equivalent antioxidant activity (TEAC)) were determined. Argentinean samples presented better activity in the DPPH and FRAP assays. Best cytoprotection against oxidative stress induced by H2O2 in AGS cells was found in one Argentinean sample at 500 μg mL(-1) (65.7%). HPLC MS/MS analysis allowed the tentative identification of 59 constituents, including eight anthocyanins, 11 conjugates of caffeic-, ferulic-, and coumaric acid, and 38 flavonoids, most of them quercetin and kaempferol derivatives. Argentinean samples showed a more complex pattern of anthocyanins, hydroxycinnamic acids (HCA), and flavonoids. Cyanidin rhamnoside hexoside and cyanidin hexoside were the main anthocyanins, accounting for 35 and 55% for the Argentinean and 60 and 27% for the ripe Chilean fruits. HCA content was about three times higher in Argentinean samples. The phenolic profiles of Chilean and Argentinean Ribes magellanicum show remarkable differences in chemical composition with higher HCA and flavonoid content in Argentinean samples.
-
Protein carriers of conjugate vaccines
Pichichero, Michael E
2013-01-01
The immunogenicity of polysaccharides as human vaccines was enhanced by coupling to protein carriers. Conjugation transformed the T cell-independent polysaccharide vaccines of the past to T cell-dependent antigenic vaccines that were much more immunogenic and launched a renaissance in vaccinology. This review discusses the conjugate vaccines for prevention of infections caused by Hemophilus influenzae type b, Streptococcus pneumoniae, and Neisseria meningitidis. Specifically, the characteristics of the proteins used in the construction of the vaccines including CRM, tetanus toxoid, diphtheria toxoid, Neisseria meningitidis outer membrane complex, and Hemophilus influenzae protein D are discussed. The studies that established differences among and key features of conjugate vaccines including immunologic memory induction, reduction of nasopharyngeal colonization and herd immunity, and antibody avidity and avidity maturation are presented. Studies of dose, schedule, response to boosters, of single protein carriers with single and multiple polysaccharides, of multiple protein carriers with multiple polysaccharides and conjugate vaccines administered concurrently with other vaccines are discussed along with undesirable consequences of conjugate vaccines. The clear benefits of conjugate vaccines in improving the protective responses of the immature immune systems of young infants and the senescent immune systems of the elderly have been made clear and opened the way to development of additional vaccines using this technology for future vaccine products. PMID:23955057
-
Conjugated polymer zwitterions and solar cells comprising conjugated polymer zwitterions
Emrick, Todd; Russell, Thomas; Page, Zachariah; Liu, Yao
2018-06-05
A conjugated polymer zwitterion includes repeating units having structure (I), (II), or a combination thereof ##STR00001## wherein Ar is independently at each occurrence a divalent substituted or unsubstituted C3-30 arylene or heteroarylene group; L is independently at each occurrence a divalent C1-16 alkylene group, C6-30arylene or heteroarylene group, or alkylene oxide group; and R1 is independently at each occurrence a zwitterion. A polymer solar cell including the conjugated polymer zwitterion is also disclosed.
-
Antioxidants are man-made or natural substances that may prevent or delay some types of cell damage. Antioxidants are found in many foods, including fruits and ... are also available as dietary supplements. Examples of antioxidants include Beta-carotene Lutein Lycopene Selenium Vitamin A ...
-
Research study of conjugate materials; Conjugate material no chosa kenkyu
Energy Technology Data Exchange (ETDEWEB)
NONE
1998-03-01
The paper reported an introductory research on possibilities of new glass `conjugate materials.` The report took up the structure and synthetic process of conjugate materials to be researched/developed, classified them according to structural elements on molecular, nanometer and cluster levels, and introduced the structures and functions. Further, as glasses with new functions to be proposed, the paper introduced transparent and high-strength glass used for houses and vehicles, light modulation glass which realizes energy saving and optical data processing, and environmentally functional glass which realizes environmental cleaning or high performance biosensor. An initial survey was also conducted on rights of intellectual property to be taken notice of in Japan and abroad in the present situation. Reports were summed up and introduced of Osaka National Research Institute, Electrotechnical Laboratory, and National Industrial Research Institute of Nagoya which are all carrying out leading studies of conjugate materials. 235 refs., 135 figs., 6 tabs.
-
Directory of Open Access Journals (Sweden)
Mykhailo Stepanovych Reheda
2017-11-01
Full Text Available We have investigated the results of alterations in indices of pro-oxidant (conjugated diene and malondialdehyde and antioxidant (superoxide dismutase, ceruloplasmin, catalase systems in guinea pigs’ lungs under the conditions of immobilization stress. The experiment was conducted on 40 female guinea pigs weighing 0.18-0.20 kg. The animals were divided into 4 groups, each contained 10 guinea pigs: I – intact guinea pigs ( control, II–guinea pigs with model of IS on1st day of experiment;Ш–animals on 2nd day of experiment;IV- group of animals on 34th day of experimental model of IS. The results of our experimental work showed a significant accumulation of lipid peroxidation products in the lung`s tissure in different periods ( on 1st, 2nd and 34th days of immobilization stress. The state of antioxidant defence was characterized by moderate decrease of inzymes activity (superoxide dismutase, catalase and ceruloplasmin. disorders of balance between pro-oxidant and antioxidant systems couse oxidative stress development.
-
Scavenger and antioxidant properties of prenylflavones isolated from Artocarpus heterophyllus.
Ko, F N; Cheng, Z J; Lin, C N; Teng, C M
1998-07-15
The antioxidant properties of prenylflavones, isolated from Artocarpus heterophyllus Lam., was evaluated in this study. Among them, artocarpine, artocarpetin, artocarpetin A, and cycloheterophyllin diacetate and peracetate had no effect on iron-induced lipid peroxidation in rat brain homogenate. They also did not scavenge the stable free radical 1,1-diphenyl-2-picrylhydrazyl. In contrast, cycloheterophyllin and artonins A and B inhibited iron-induced lipid peroxidation in rat brain homogenate and scavenged 1,1-diphenyl-2-picrylhydrazyl. They also scavenged peroxyl radicals and hydroxyl radicals that were generated by 2,2'-azobis(2-amidinopropane) dihydrochloride and the Fe3+-ascorbate-EDTA-H2O2 system, respectively. However, they did not inhibit xanthine oxidase activity or scavenge superoxide anion, hydrogen peroxide, carbon radical, or peroxyl radicals derived from 2,2'-azobis(2,4-dimethylvaleronitrile) in hexane. Moreover, cycloheterophyllin and artonins A and B inhibited copper-catalyzed oxidation of human low-density lipoprotein, as measured by fluorescence intensity, thiobarbituric acid-reactive substance and conjugated-diene formations and electrophoretic mobility. It is concluded that cycloheterophyllin and artonins A and B serve as powerful antioxidants against lipid peroxidation when biomembranes are exposed to oxygen radicals.
-
Misonidazole-glutathione conjugates in CHO cells
International Nuclear Information System (INIS)
Varghese, A.J.; Whitmore, G.F.
1984-01-01
Misonidazole, after reduction to the hydroxylamine derivative, reacts with glutathione (GSH) under physiological conditions. The reaction product has been identified as a mixture of two isomeric conjugates. When water soluble extracts of CHO cells exposed to misonidazole under hypoxic conditions are subjected to HPLC analysis, misonidazole derivatives, having the same chromatographic properties as the GSH-MISO conjugates, were detected. When CHO cells were incubated with misonidazole in the presence of added GSH, a substantial increase in the amount of the conjugate was detected. When extracts of CHO cells exposed to misonidazole under hypoxia were subsequently exposed to GSH, an increased formation of the conjugate was observed. A rearrangement product of the hydroxylamine derivative of misonidazole is postulated as the reactive intermediate responsible for the formation of the conjugate
-
Modelling conjugation with stochastic differential equations
DEFF Research Database (Denmark)
Philipsen, Kirsten Riber; Christiansen, Lasse Engbo; Hasman, Henrik
2010-01-01
Enterococcus faecium strains in a rich exhaustible media. The model contains a new expression for a substrate dependent conjugation rate. A maximum likelihood based method is used to estimate the model parameters. Different models including different noise structure for the system and observations are compared......Conjugation is an important mechanism involved in the transfer of resistance between bacteria. In this article a stochastic differential equation based model consisting of a continuous time state equation and a discrete time measurement equation is introduced to model growth and conjugation of two...... using a likelihood-ratio test and Akaike's information criterion. Experiments indicating conjugation on the agar plates selecting for transconjugants motivates the introduction of an extended model, for which conjugation on the agar plate is described in the measurement equation. This model is compared...
-
Institute of Scientific and Technical Information of China (English)
Ademola Ayeleso; Nicole Brooks; Oluwafemi Oguntibeju
2014-01-01
Objective:To investigate the role of red palm oil(RPO), rooibos tea extract(RTE) and their combined treatment(RPO+RTE) on antioxidant status in streptozotocin(STZ)-induced diabetic rats.Methods:Diabetes mellitus was induced by a single administration of streptozotocin(50 mg/kg) and the rats were treated for7 weeks.Antioxidant enzymes [catalase(CAT), glutathione peroxidase (GPx), superoxide dismutase(SOD)], antioxidant capacity [trolox equivalence antioxidant capacity (TEAC), oxygen radical absorbance capacity(ORAC)] as well as total protein, albumin, globulin, total glutathione, conjugated diene and thiobarbituric acid reactive substances(TBARS) were investigated.Results:Treatment withRPO,RTE andRPO+RTE significantly(p>0.05) improved liverSOD and plasmaORAC in the diabetic rats.Similarly, diabetic rats treated withRTE and RPO+RTE enhanced liverGPx.A significant(P<0.05) increase in the plasmaTBARS in the diabetic control group was observed when compared with the normal control group.Treatment of diabetic rats withRTE andRPO+RTE reduced plasmaTBARS to a level not significantly different atP<0.05 from the normal control group. Conclusions:The results revealed the anti-oxidative potentials of red palm oil, rooibos and their combination in diabetic conditions and hence, they could be useful in the management of diabetes and its complications.
-
Fan, Yuting; Yi, Jiang; Zhang, Yuzhu; Yokoyama, Wallace
2018-01-15
Bovine serum albumin (BSA)-dextran conjugate was prepared with glycation. Self-assembly nanoparticles were synthesized with a green, and facile approach. The effects of dry-heating time on the fabrication and characteristics of BSA-dextran conjugate nanoparticles were examined. Stable nanoparticles (dextran was grafted onto the BSA to provide significant steric hindrance. Particle size decreased with the increase of dry-heating time and the lowest particle size (51.2nm) was obtained after 24h dry-heating. The nanoparticles were stable in a wide pH range (pH 2.0-7.0). The particle size of nanoparticles increased to 115nm after curcumin incorporation and was stable even after one-month storage. TEM results demonstrated that curcumin-loaded nanoparticles displayed a spherical structure and were homogeneously dispersed. Curcumin in BSA-dextran nanoparticle showed better stability, compared to free curcumin. In addition, BSA-dextran nanoparticles can improve the cellular antioxidant activity of curcumin in Caco-2 cells. Copyright © 2017 Elsevier Ltd. All rights reserved.
-
Poly(2-oxazoline)-Antibiotic Conjugates with Penicillins.
Schmidt, Martin; Bast, Livia K; Lanfer, Franziska; Richter, Lena; Hennes, Elisabeth; Seymen, Rana; Krumm, Christian; Tiller, Joerg C
2017-09-20
The conjugation of antibiotics with polymers is rarely done, but it might be a promising alternative to low-molecular-weight derivatization. The two penicillins penicillin G (PenG) and penicillin V (PenV) were attached to the end groups of different water-soluble poly(2-oxazoline)s (POx) via their carboxylic acid function. This ester group was shown to be more stable against hydrolysis than the β-lactam ring of the penicillins. The conjugates are still antimicrobially active and up to 20 times more stable against penicillinase catalyzed hydrolysis. The antibiotic activity of the conjugates against Staphylococcus aureus in the presence of penicillinase is up to 350 times higher compared with the free antibiotics. Conjugates with a second antimicrobial function, a dodecyltrimethylammonium group (DDA-X), at the starting end of the PenG and PenV POx conjugates are more antimicrobially active than the conjugates without DDA-X and show high activity in the presence of penicillinase. For example, the conjugates DDA-X-PEtOx-PenG and DDA-X-PEtOx-PenV are 200 to 350 times more active against S. aureus in the presence of penicillinase and almost as effective as the penicillinase stable cloxacollin (Clox) under these conditions. These conjugates show even greater activity compared to cloxacollin without this enzyme present. Further, both conjugates kill Escherichia coli more effectively than PenG and Clox.
-
Approximate error conjugation gradient minimization methods
Kallman, Jeffrey S
2013-05-21
In one embodiment, a method includes selecting a subset of rays from a set of all rays to use in an error calculation for a constrained conjugate gradient minimization problem, calculating an approximate error using the subset of rays, and calculating a minimum in a conjugate gradient direction based on the approximate error. In another embodiment, a system includes a processor for executing logic, logic for selecting a subset of rays from a set of all rays to use in an error calculation for a constrained conjugate gradient minimization problem, logic for calculating an approximate error using the subset of rays, and logic for calculating a minimum in a conjugate gradient direction based on the approximate error. In other embodiments, computer program products, methods, and systems are described capable of using approximate error in constrained conjugate gradient minimization problems.
-
Chitosan derivatives with antimicrobial, antitumour and antioxidant activities--a review.
Jarmila, Vinsová; Vavríková, Eva
2011-01-01
Chitosan is a linear polysaccharide with a good biodegradability, biocompatibility, and no toxicity, which provide it with huge potential for future development. The chitosan molecule appears to be a suitable polymeric complex for many biomedical applications. This review gathers current findings on the antibacterial, antifungal, antitumour and antioxidant activities of chitosan derivatives and concurs with our previous review presenting data collected up to 2008. Antibacterial activity is based on molecular weight, the degree of deacetylation, the type of substitutents, which can be cationic or easily form cations, and the type of bacterium. In general, high molecular weight chitosan cannot pass through cell membranes and forms a film that protects cells against nutrient transport through the microbial cell membrane. Low molecular weight chitosan derivatives are water soluble and can better incorporate the active molecule into the cell. Gram-negative bacteria, often represented by Escherichia coli, have an anionic bacterial surface on which cationic chitosan derivatives interact electrostatically. Thus, many chitosan conjugates have cationic components such as ammonium, pyridinium or piperazinium substituents introduced into their molecules to increase their positive charge. Gram-positive bacteria like Staphylococcus aureus are inhibited by the binding of lower molecular weight chitosan derivatives to DNA or RNA. Chitosan nanoparticles exhibit an increase in loading capacity and efficacy. Antitumour active compounds such as doxorubicin, paclitaxel, docetaxel and norcantharidin are used as drug carriers. It is evident that chitosan, with its low molecular weight, is a useful carrier for molecular drugs requiring targeted delivery. The antioxidant scavenging activity of chitosan has been established by the strong hydrogen-donating ability of chitosan. The low molecular weight and greater degree of quarternization have a positive influence on the antioxidant activity
-
Relationships among alcoholic liver disease, antioxidants, and antioxidant enzymes.
Han, Kyu-Ho; Hashimoto, Naoto; Fukushima, Michihiro
2016-01-07
Excessive consumption of alcoholic beverages is a serious cause of liver disease worldwide. The metabolism of ethanol generates reactive oxygen species, which play a significant role in the deterioration of alcoholic liver disease (ALD). Antioxidant phytochemicals, such as polyphenols, regulate the expression of ALD-associated proteins and peptides, namely, catalase, superoxide dismutase, glutathione, glutathione peroxidase, and glutathione reductase. These plant antioxidants have electrophilic activity and may induce antioxidant enzymes via the Kelch-like ECH-associated protein 1-NF-E2-related factor-2 pathway and antioxidant responsive elements. Furthermore, these antioxidants are reported to alleviate cell injury caused by oxidants or inflammatory cytokines. These phenomena are likely induced via the regulation of mitogen-activating protein kinase (MAPK) pathways by plant antioxidants, similar to preconditioning in ischemia-reperfusion models. Although the relationship between plant antioxidants and ALD has not been adequately investigated, plant antioxidants may be preventive for ALD because of their electrophilic and regulatory activities in the MAPK pathway.
-
Li, Chunju; Lin, Qixun; Wang, Jun; Shen, Lijuan; Ma, Guanghui; Su, Zhiguo; Hu, Tao
2012-12-31
Ribonuclease A (RNase A) is a therapeutic enzyme with cytotoxic action against tumor cells. Its clinical application is limited by the short half-life and insufficient stability. Conjugation of albumin can overcome the limitation, whereas dramatically decrease the enzymatic activity of RNase A. Here, three strategies were proposed to prepare the RNase A-bovine serum albumin (BSA) conjugates. R-SMCC-B (a conjugate of four RNase A attached with one BSA) and R-PEG-B (a mono-conjugate) were prepared using Sulfo-SMCC (a short bifunctional linker) and mal-PEG-NHS (a bifunctional PEG), respectively. Mal-PEG-NHS and hexadecylamine (HDA) were used to prepare the mono-conjugate, R-HDA-B, where HDA was adopted to bind BSA. The PEG linker can elongate the proximity between RNase A and BSA. In contrast, four RNase A were closely located on BSA in R-SMCC-B. R-SMCC-B showed the lowest K(m) and the highest relative enzymatic activity and k(cat)/K(m) in the three conjugates. Presumably, the tetravalent interaction of RNase A in R-SMCC-B can increase the binding affinity to its substrate. In addition, the slow release of BSA from R-HDA-B may increase the enzymatic activity of R-HDA-B. Our study is expected to provide strategies to develop protein-albumin conjugate with high therapeutic potential. Copyright © 2012 Elsevier B.V. All rights reserved.
-
Activated Microglia Targeting Dendrimer-Minocycline Conjugate as Therapeutics for Neuroinflammation.
Sharma, Rishi; Kim, Soo-Young; Sharma, Anjali; Zhang, Zhi; Kambhampati, Siva Pramodh; Kannan, Sujatha; Kannan, Rangaramanujam M
2017-11-15
Brain-related disorders have outmatched cancer and cardiovascular diseases worldwide as the leading cause of morbidity and mortality. The lack of effective therapies and the relatively dry central nervous system (CNS) drug pipeline pose formidable challenge. Superior, targeted delivery of current clinically approved drugs may offer significant potential. Minocycline has shown promise for the treatment of neurological diseases owing to its ability to penetrate the blood-brain barrier (BBB) and potency. Despite its potential in the clinic and in preclinical models, the high doses needed to affect a positive therapeutic response have led to side effects. Targeted delivery of minocycline to the injured site and injured cells in the brain can be highly beneficial. Systemically administered hydroxyl poly(amidoamine) (PAMAM) generation-6 (G6) dendrimers have a longer blood circulation time and have been shown to cross the impaired BBB. We have successfully prepared and characterized the in vitro efficacy and in vivo targeting ability of hydroxyl-G6 PAMAM dendrimer-9-amino-minocycline conjugate (D-mino). Minocycline is a challenging drug to carry out chemical transformations due to its inherent instability. We used a combination of a highly efficient and mild copper catalyzed azide-alkyne click reaction (CuAAC) along with microwave energy to conjugate 9-amino-minocycline (mino) to the dendrimer surface via enzyme responsive linkages. D-mino was further evaluated for anti-inflammatory and antioxidant activity in lipopolysaccharides-activated murine microglial cells. D-mino conjugates enhanced the intracellular availability of the drug due to their rapid uptake, suppressed inflammatory cytokine tumor necrosis factor α (TNF-α) production, and reduced oxidative stress by suppressing nitric oxide production, all significantly better than the free drug. Fluorescently labeled dendrimer conjugate (Cy5-D-mino) was systematically administered (intravenous, 55 mg/kg) on postnatal
-
Chen, Tao; Su, Dian; Gruenhagen, Jason; Gu, Christine; Li, Yi; Yehl, Peter; Chetwyn, Nik P; Medley, Colin D
2016-01-05
Antibody-drug conjugates (ADCs) offer new therapeutic options for advanced cancer patients through precision killing with fewer side effects. The stability and efficacy of ADCs are closely related, emphasizing the urgency and importance of gaining a comprehensive understanding of ADC stability. In this work, a chemical de-conjugation approach was developed to investigate the in-situ stability of the small molecule drug while it is conjugated to the antibody. This method involves chemical-mediated release of the small molecule drug from the ADC and subsequent characterization of the released small molecule drug by HPLC. The feasibility of this technique was demonstrated utilizing a model ADC containing a disulfide linker that is sensitive to the reducing environment within cancer cells. Five reducing agents were screened for use in de-conjugation; tris(2-carboxyethyl) phosphine (TCEP) was selected for further optimization due to its high efficiency and clean impurity profile. The optimized de-conjugation assay was shown to have excellent specificity and precision. More importantly, it was shown to be stability indicating, enabling the identification and quantification of the small molecule drug and its degradation products under different formulation pHs and storage temperatures. In summary, the chemical de-conjugation strategy demonstrated here offers a powerful tool to assess the in-situ stability of small molecule drugs on ADCs and the resulting information will shed light on ADC formulation/process development and storage condition selection. Copyright © 2015 Elsevier B.V. All rights reserved.
-
Photoluminescence in conjugated polymers
International Nuclear Information System (INIS)
Furst, J.E.; Laugesen, R.; Dastoor, P.; McNeill, C.
2002-01-01
Full text: Conjugated polymers combine the electronic and optical properties of semiconductors with the processability of polymers. They contain a sequence of alternate single and double carbon bonds so that the overlap of unhybridised p z orbitals creates a delocalised ρ system which gives semiconducting properties with p-bonding (valence) and p* -antibonding (conduction) bands. Photoluminesence (PL) in conjugated polymers results from the radiative decay of singlet excitons confined to a single chain. The present work is the first in a series of studies in our laboratory that will characterize the optical properties of conjugated polymers. The experiment involves the illumination of thin films of conjugated polymer with UV light (I=360 nm) and observing the subsequent fluorescence using a custom-built, fluorescence spectrometer. Photoluminesence spectra provide basic information about the structure of the polymer film. A typical spectrum is shown in the accompanying figure. The position of the first peak is related to the polymer chain length and resolved multiple vibronic peaks are an indication of film structure and morphology. We will also present results related to the optical degradation of these materials when exposed to air and UV light
-
Bio-Conjugates for Nanoscale Applications
DEFF Research Database (Denmark)
Villadsen, Klaus
Bio-conjugates for Nanoscale Applications is the title of this thesis, which covers three different projects in chemical bio-conjugation research, namely synthesis and applications of: Lipidated fluorescent peptides, carbohydrate oxime-azide linkers and N-aryl O-R2 oxyamine derivatives. Lipidated...
-
Tang, Yao; Li, Xihong; Zhang, Bing; Chen, Peter X; Liu, Ronghua; Tsao, Rong
2015-01-01
Quinoa (Chenopodium quinoa Willd.) is known for its exceptional nutritional value and potential health benefits. The present study identified the composition of different forms of extractable phenolics and betacyanins of quinoa cultivars in white, red and black, and how they contribute to antioxidant activities. Results showed that at least 23 phenolic compounds were found in either free or conjugated forms (liberated by alkaline and/or acid hydrolysis); the majority of which were phenolic acids, mainly vanillic acid, ferulic acid and their derivatives as well as main flavonoids quercetin, kaempferol and their glycosides. Betacyanins, mainly betanin and isobetanin, were confirmed for the first time to be the pigments of the red and black quinoa seeds, instead of anthocyanins. Darker quinoa seeds had higher phenolic concentration and antioxidant activity. Findings of these phenolics, along with betacyanins in this study add new knowledge to the functional components of quinoa seeds of different cultivar background. Crown Copyright © 2014. Published by Elsevier Ltd. All rights reserved.
-
Lima, Igo T.; Risko, Chad; Aziz, Saadullah Gary; Da Silva Filho, Demé trio A Da Silva; Bredas, Jean-Luc
2014-01-01
Donor-acceptor π-conjugated copolymers are of interest for a wide range of electronic applications, including field-effect transistors and solar cells. Here, we present a density functional theory (DFT) study of the impact of varying the conjugation pathway on the geometric, electronic, and optical properties of donor-acceptor systems. We consider both linear ("in series"), traditional conjugation among the donor-acceptor moieties versus structures where the acceptor units are appended orthogonally to the linear, donor-only conjugated backbone. Long-range-corrected hybrid functionals are used in the investigation with the values of the tuned long-range separation parameters providing an estimate of the extent of conjugation as a function of the oligomer architecture. Considerable differences in the electronic and optical properties are determined as a function of the nature of the conjugation pathway, features that should be taken into account in the design of donor-acceptor copolymers.
-
Jiang, Hongqin; Wang, Zhenzhen; Ma, Yong; Qu, Yanghua; Lu, Xiaonan; Luo, Hailing
2015-07-01
Lycopene, a red non-provitamin A carotenoid, mainly presenting in tomato and tomato byproducts, has the highest antioxidant activity among carotenoids because of its high number of conjugated double bonds. The objective of this study was to investigate the effect of lycopene supplementation in the diet on plasma lipid profile, lipid peroxidation and antioxidant defense system in feedlot lamb. Twenty-eight Bamei male lambs (90 days old) were divided into four groups and fed a basal diet (LP0, 40:60 roughage: concentrate) or the basal diet supplemented with 50, 100, and 200 mg/kg lycopene. After 120 days of feeding, all lambs were slaughtered and sampled. Dietary lycopene supplementation significantly reduced the levels of plasma total cholesterol (p0.05). The levels of TG (pCAT, pCAT (p<0.05, linearly) and SOD (p<0.001, linearly). Therefore, it was concluded that lycopene supplementation improved the antioxidant status of the lamb and optimized the plasma lipid profile, the dosage of 200 mg lycopene/kg feed might be desirable for growing lambs to prevent environment stress and maintain normal physiological metabolism.
-
Association of antioxidant nutraceuticals and acetaminophen (paracetamol): Friend or foe?
Abdel-Daim, Mohamed; Abushouk, Abdelrahman Ibrahim; Reggi, Raffaella; Yarla, Nagendra Sastry; Palmery, Maura; Peluso, Ilaria
2018-04-01
Acetaminophen (paracetamol or APAP) is an analgesic and antipyretic drug that can induce oxidative stress-mediated hepatotoxicity at high doses. Several studies reported that antioxidant nutraceuticals, in particular phenolic phytochemicals from dietary food, spices, herbs and algae have hepatoprotective effects. Others, however, suggested that they may negatively impact the metabolism, efficacy and toxicity of APAP. The aim of this review is to discuss the pros and cons of the association of antioxidant nutraceuticals and APAP by reviewing the in vivo evidence, with particular reference to APAP pharmacokinetics and hepatotoxicity. Results from the murine models of APAP-induced hepatotoxicity showed amelioration of liver damage with nutraceuticals coadministration, as well as reductions in tissue markers of oxidative stress, and serum levels of hepatic enzymes, bilirubin, cholesterol, triglycerides and inflammatory cytokines. On the other hand, both increased and decreased APAP plasma levels have been reported, depending on the nutraceutical type and route of administration. For example, studies showed that repeated administration of flavonoids causes down-regulation of cytochrome P450 enzymes and up-regulation of uridine diphosphate glucuronosyltransferases (UGT). Moreover, nutraceuticals can alter the levels of APAP metabolites, such as mercapturate glucuronide, sulfate and cysteine conjugates. Overall, the reviewed in vivo studies indicate that interactions between APAP and nutraceuticals or plant foods exist. However, the majority of data come from animal models with doses of phytochemicals far from dietary ones. Human studies should investigate gene-diet interactions, as well as ethnic variability in order to clarify the pros and cons of co-administering antioxidant nutraceuticals and APAP. Copyright © 2017. Published by Elsevier B.V.
-
Organometallic B12-DNA conjugate
DEFF Research Database (Denmark)
Hunger, Miriam; Mutti, Elena; Rieder, Alexander
2014-01-01
Design, synthesis, and structural characterization of a B12-octadecanucleotide are presented herein, a new organometallic B12-DNA conjugate. In such covalent conjugates, the natural B12 moiety may be a versatile vector for controlled in vivo delivery of oligonucleotides to cellular targets in hum...
-
The Conjugate Acid-Base Chart.
Treptow, Richard S.
1986-01-01
Discusses the difficulties that beginning chemistry students have in understanding acid-base chemistry. Describes the use of conjugate acid-base charts in helping students visualize the conjugate relationship. Addresses chart construction, metal ions, buffers and pH titrations, and the organic functional groups and nonaqueous solvents. (TW)
-
Total synthesis of (-)- and (+)-tedanalactam
Digital Repository Service at National Institute of Oceanography (India)
Majik, M.S.; Parameswaran, P.S.; Tilve, S.G.
: The Journal of Organic Chemistry, vol.74(16); 6378-6381 1 Total Synthesis of (-) and (+)-Tedanalactam Mahesh S. Majik, † Peruninakulath S. Parameswaran, ‡ and Santosh G. Tilve* ,† Department of Chemistry, Goa University, Taleigao Plateau, Goa 403..., displaying a wide range of biological activities. 1 Piperidones are key synthetic intermediates 2 for the synthesis of piperidine ring due to the presence of keto function which allows the introduction of other groups. Piperidones are also known...
-
REVIEW ARTICLE Conjugated Hyperbilirubinaemia in Early Infancy ...
African Journals Online (AJOL)
REVIEW ARTICLE Conjugated Hyperbilirubinaemia in Early Infancy. AOK Johnson. Abstract. Conjugated hyperbilirubinaemia exists when the conjugated serum bilirubin level is more than 2 mg/dl or more than 20 per cent of the total serum bilirubin. It is always pathological in early infancy. The causes are many and diverse ...
-
Application of optical phase conjugation to plasma diagnostics (invited)
International Nuclear Information System (INIS)
Jahoda, F.C.; Anderson, B.T.; Forman, P.R.; Weber, P.G.
1985-01-01
Several possibilities for plasma diagnostics provided by optical phase conjugation and, in particular, self-pumped phase conjugation in barium titanate (BaTiO 3 ) are discussed. These include placing a plasma within a dye laser cavity equipped with a phase conjugate mirror for intracavity absorption measurements, time differential refractometry with high spatial resolution, and simplified real-time holographic interferometry. The principles of phase conjugation with particular reference to photorefractive media and the special advantages of self-pumped phase conjugation are reviewed prior to the discussion of the applications. Distinctions are made in the applications between those for which photorefractive conjugators are essential and those for which they only offer experimental simplification relative to other types of phase conjugators
-
Wu, Douglas C; Goldman, Mitchel P
2017-10-01
Background: Fractionated, ablative lasers are usually associated with post-treatment erythema, edema, and crusting, which can last from 5 to 14 days. Conjugated linolenic acid, an omega-5 fatty acid, has significant antioxidant and anti-inflammatory properties, and has been shown to stimulate keratinocyte proliferation and epidermal regeneration. By modulating the early inflammatory milieu and directly affecting skin structure and function, conjugated linolenic acid might therefore shorten downtime following fractionated ablative laser resurfacing of the face. Objective: To evaluate the efficacy and subject satisfaction of a topical regimen containing conjugated linolenic acid derived from pomegranate seed extract in accelerating wound healing and improving skin quality following fractionated ablative laser resurfacing of the face. Materials and Methods: Thirty-four subjects were enrolled and received fractionated CO2 laser resurfacing. Subjects were randomized to use the test healing regimen (n=24) or 1% dimethicone ointment (n=10) post-procedure. The primary endpoint was the degree of erythema, edema, crusting, and exudation evaluated by a blinded clinician at post-treatment Days 1,3,7,10, 14, and 30. Secondary endpoints included a blinded evaluator assessment of the degree of wrinkling and elastosis using the Fitzpatrick-Goldman Wrinkle and Elastosis Scale; subject-assessed degree of pain, itching, tightness, oozing, and crusting; and subject overall satisfaction. Results: Subjects who applied the topical conjugated linolenic acid healing regimen experienced significantly reduced edema on post-procedure Day 3 ( p =0.04), and itching on Days 1 and 3 ( p =0.03 and p =0.04). Both regimens produced significant improvements in wrinkling and elastosis at Days 14 and 30 post-treatment, with conjugated linolenic acid outperforming placebo in improvements in wrinkling at Day 14. Both regimens were well tolerated with no statistical differences in adverse events or
-
Obermeier, Michael; Schröder, Christian A; Helmreich, Brigitte; Schröder, Peter
2015-12-01
Lemna minor L., a widely used model plant for toxicity tests has raised interest for its application to phytoremediation due to its rapid growth and ubiquitous occurrence. In rural areas, the pollution of water bodies with heavy metals and agrochemicals poses a problem to surface water quality. Among problematic compounds, heavy metals (copper) and pesticides are frequently found in water bodies. To establish duckweed as a potential plant for phytoremediation, enzymatic and antioxidative stress responses of Lemna minor during exposure to copper and a chloroacetamide herbicide were investigated in laboratory studies. The present study aimed at evaluating growth and the antioxidative and glutathione-dependent enzyme activity of Lemna plants and its performance in a scenario for phytoremediation of copper and a chloroacetamide herbicide. Lemna minor was grown in Steinberg medium under controlled conditions. Plants were treated with CuSO4 (ion conc. 50 and 100 μg/L) and pethoxamide (1.25 and 2.5 μg/L). Measurements following published methods focused on plant growth, oxidative stress, and basic detoxification enzymes. Duckweed proved to survive treatment with the respective concentrations of both pollutants very well. Its growth was inhibited scarcely, and no visible symptoms occurred. On the cellular basis, accumulation of O2(-) and H2O2 were detected, as well as stress reactions of antioxidative enzymes. Duckweed detoxification potential for organic pollutants was high and increased significantly with incubation. Pethoxamide was found to be conjugated with glutathione. Copper was accumulated in the fronds at high levels, and transient oxidative defense reactions were triggered. This work confirms the significance of L. minor for the removal of copper from water and the conjugation of the selective herbicide pethoxamide. Both organic and inorganic xenobiotics induced different trends of enzymatic and antioxidative stress response. The strong increase of stress
-
Feidantsis, Konstantinos; Anestis, Andreas; Michaelidis, Basile
2013-10-01
In the present work we investigated the seasonal variations of apoptotic and antioxidant proteins in the heart and gastrocnemius muscle of the amphibian Pelophylax ridibundus. Particularly processes studied included the evaluation of hypoxia through the levels of transcriptional factor Hif-1α, of apoptosis through the determination of Bcl-2 and Bax, ubiquitin conjugates levels and the antioxidant defense through the determination of the activity of enzymes such as superoxide dismutase, catalase and glutathione peroxidase. Due to a general metabolic depression during overwintering, levels of the above mentioned proteins and enzymes are generally retained at low levels of expression and activity in the examined tissues of P. ridibundus. On the other hand recovery from overwintering induces oxidative stress, followed by increased levels of the specific proteins and enzymes. A milder up-regulation of antioxidant enzymes during overwintering probably prepares P. ridibundus for oxidative stress during arousal. The seasonal activation of these mechanisms seems to protect this species from these unfavourable conditions. Copyright © 2013 Elsevier Inc. All rights reserved.
-
Antioxidant Capacities of Fractions of Bamboo Shaving Extract and Their Antioxidant Components.
Gong, Jinyan; Huang, Jun; Xiao, Gongnian; Chen, Feng; Lee, Bolim; Ge, Qing; You, Yuru; Liu, Shiwang; Zhang, Ying
2016-07-30
This research was conducted for evaluation of antioxidant activities of four fractions from bamboo shavings extract (BSE) and their antioxidant components. The antioxidant capacities of BSE and four fractions on ABTS, DPPH, FRAP and total antioxidant capacity assays exhibited the following descending order: DF > n-butanol fraction (BF) > BSE ≈ ethyl acetate fraction (AF) > water fraction (WF). Among the identified phenolic compounds, caffeic acid exhibited the highest antioxidant capacities on DPPH, FRAP and total antioxidant capacity assays. An extremely significant positive correlation between the antioxidant activities with the contents of total flavonoids, total phenolic acids, or total phenolics was observed in this study. The result indicated that the bamboo shaving extract and its solvent fractions could act as natural antioxidants in light of their potent antioxidant activities.
-
Methotrexate and epirubicin conjugates as potential antitumor drugs
Directory of Open Access Journals (Sweden)
Szymon Wojciech Kmiecik
2017-07-01
Full Text Available Introduction: The use of hybrid molecules has become one of the most significant approaches in new cytotoxic drug design. This study describes synthesis and characterization of conjugates consisting of two well-known and characterized chemotherapeutic agents: methotrexate (MTX and epirubicin (EPR. The synthesized conjugates combine two significant anticancer strategies: combinatory therapy and targeted therapy. These two drugs were chosen because they have different mechanisms of action, which can increase the anticancer effect of the obtained conjugates. MTX, which is a folic acid analog, has high cytotoxic properties and can serve as a targeting moiety that can reach folate receptors (FRs overexpresing tumor cells. Combination of nonselective drugs such as EPR with MTX can increase the selectivity of the obtained conjugates, while maintaining the high cytotoxic properties.Materials and methods: Conjugates were purified by RP-HPLC and the structure was investigated by MS and MS/MS methods. The effect of the conjugates on proliferation of LoVo, LoVo/Dx, MCF-7 and MV-4-11 human cancer cell lines was determined by SRB or MTT assay.Results: The conjugation reaction results in the formation of monosubstituted (α, γ and disubstituted MTX derivatives. In vitro proliferation data demonstrate that the conjugates synthesized in our study show lower cytotoxic properties than both chemotherapeutics used alone.Discussion: Epirubicin cytotoxicity was not observed in obtained conjugates. Effective drugs release after internalization needs further investigation.
-
Directory of Open Access Journals (Sweden)
Sujiet Puthenveetil
Full Text Available Antibody drug conjugates (ADCs are no longer an unknown entity in the field of cancer therapy with the success of marketed ADCs like ADCETRIS and KADCYLA and numerous others advancing through clinical trials. The pursuit of novel cytotoxic payloads beyond the mictotubule inhibitors and DNA damaging agents has led us to the recent discovery of an mRNA splicing inhibitor, thailanstatin, as a potent ADC payload. In our previous work, we observed that the potency of this payload was uniquely tied to the method of conjugation, with lysine conjugates showing much superior potency as compared to cysteine conjugates. However, the ADC field is rapidly shifting towards site-specific ADCs due to their advantages in manufacturability, characterization and safety. In this work we report the identification of a highly efficacious site-specific thailanstatin ADC. The site of conjugation played a critical role on both the in vitro and in vivo potency of these ADCs. During the course of this study, we developed a novel methodology of loading a single site with multiple payloads using an in situ generated multi-drug carrying peptidic linker that allowed us to rapidly screen for optimal conjugation sites. Using this methodology, we were able to identify a double-cysteine mutant ADC delivering four-loaded thailanstatin that was very efficacious in a gastric cancer xenograft model at 3mg/kg and was also shown to be efficacious against T-DM1 resistant and MDR1 overexpressing tumor cell lines.
-
Puthenveetil, Sujiet; He, Haiyin; Loganzo, Frank; Musto, Sylvia; Teske, Jesse; Green, Michael; Tan, Xingzhi; Hosselet, Christine; Lucas, Judy; Tumey, L Nathan; Sapra, Puja; Subramanyam, Chakrapani; O'Donnell, Christopher J; Graziani, Edmund I
2017-01-01
Antibody drug conjugates (ADCs) are no longer an unknown entity in the field of cancer therapy with the success of marketed ADCs like ADCETRIS and KADCYLA and numerous others advancing through clinical trials. The pursuit of novel cytotoxic payloads beyond the mictotubule inhibitors and DNA damaging agents has led us to the recent discovery of an mRNA splicing inhibitor, thailanstatin, as a potent ADC payload. In our previous work, we observed that the potency of this payload was uniquely tied to the method of conjugation, with lysine conjugates showing much superior potency as compared to cysteine conjugates. However, the ADC field is rapidly shifting towards site-specific ADCs due to their advantages in manufacturability, characterization and safety. In this work we report the identification of a highly efficacious site-specific thailanstatin ADC. The site of conjugation played a critical role on both the in vitro and in vivo potency of these ADCs. During the course of this study, we developed a novel methodology of loading a single site with multiple payloads using an in situ generated multi-drug carrying peptidic linker that allowed us to rapidly screen for optimal conjugation sites. Using this methodology, we were able to identify a double-cysteine mutant ADC delivering four-loaded thailanstatin that was very efficacious in a gastric cancer xenograft model at 3mg/kg and was also shown to be efficacious against T-DM1 resistant and MDR1 overexpressing tumor cell lines.
-
Rybicka, Marta; Stachowska, Ewa; Gutowska, Izabela; Parczewski, Miłosz; Baśkiewicz, Magdalena; Machaliński, Bogusław; Boroń-Kaczmarska, Anna; Chlubek, Dariusz
2011-04-27
The aim of this study was to investigate the effect of conjugated linoleic acids (CLAs) on macrophage reactive oxygen species synthesis and the activity and expression of antioxidant enzymes, catalase (Cat), glutathione peroxidase (GPx), and superoxide dismutase (SOD). The macrophages were obtained from the THP-1 monocytic cell line. Cells were incubated with the addition of cis-9,trans-11 CLA or trans-10,cis-12 CLA or linoleic acid. Reactive oxygen species (ROS) formation was estimated by flow cytometry. Enzymes activity was measured spectrophotometrically. The antioxidant enzyme mRNA expression was estimated by real-time reverse transcriptase polymerase chain reaction (RT-PCR). Statistical analysis was based on nonparametric statistical tests [Friedman analysis of variation (ANOVA) and Wilcoxon signed-rank test]. cis-9,trans-11 CLA significantly increased the activity of Cat, while trans-10,cis-12 CLA notably influenced GPx activity. Both isomers significantly decreased mRNA expression for Cat. Only trans-10,cis-12 significantly influenced mRNA for SOD-2 expression. The CLAs activate processes of the ROS formation in macrophages. Adverse metabolic effects of each isomer action were observed.
-
In Vitro Evaluation of Third Generation PAMAM Dendrimer Conjugates
Directory of Open Access Journals (Sweden)
Mohammad Najlah
2017-10-01
Full Text Available The present study compares the use of high generation G3 and low generation G0 Polyamidoamine (PAMAM dendrimers as drug carriers of naproxen (NAP, a poorly water soluble drug. Naproxen was conjugated to G3 in different ratios and to G0 in a 1:1 ratio via a diethylene glycol linker. A lauroyl chain (L, a lipophilic permeability enhancer, was attached to G3 and G0 prodrugs. The G3 and G0 conjugates were more hydrophilic than naproxen as evaluated by the measurement of partitioning between 1-octanol and a phosphate buffer at pH 7.4 and pH 1.2. The unmodified surface PAMAM-NAP conjugates showed significant solubility enhancements of NAP at pH 1.2; however, with the number of NAP conjugated to G3, this was limited to 10 molecules. The lactate dehydrogenase (LDH assay indicated that the G3 dendrimer conjugates had a concentration dependent toxicity towards Caco-2 cells. Attaching naproxen to the surface of the dendrimer increased the IC50 of the resulting prodrugs towards Caco-2 cells. The lauroyl G3 conjugates showed the highest toxicity amongst the PAMAM dendrimer conjugates investigated and were significantly more toxic than the lauroyl-G0-naproxen conjugates. The permeability of naproxen across monolayers of Caco-2 cells was significantly increased by its conjugation to either G3 or G0 PAMAM dendrimers. Lauroyl-G0 conjugates displayed considerably lower cytotoxicity than G3 conjugates and may be preferable for use as a drug carrier for low soluble drugs such as naproxen.
-
Modified Folin-Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants.
Berker, Kadriye Isil; Ozdemir Olgun, F Ayca; Ozyurt, Dilek; Demirata, Birsen; Apak, Resat
2013-05-22
The Folin-Ciocalteu (FC) method of performing a total phenolics assay, originally developed for protein determination, has recently evolved as a total antioxidant capacity assay but was found to be incapable of measuring lipophilic antioxidants due to the high affinity of the FC chromophore, that is, multivalent-charged phospho-tungsto-molybdate(V), toward water. Thus, the FC method was modified and standardized so as to enable simultaneous measurement of lipophilic and hydrophilic antioxidants in NaOH-added isobutanol-water medium. Optimal conditions were as follows: dilution ratio of aqueous FC reagent with iso-BuOH (1:2, v/v), final NaOH concentration of 3.5 × 10(-2) M, reaction time of 20 min, and maximum absorption wavelength of 665 nm. The modified procedure was successfully applied to the total antioxidant capacity assay of trolox, quercetin, ascorbic acid, gallic acid, catechin, caffeic acid, ferulic acid, rosmarinic acid, glutathione, and cysteine, as well as of lipophilic antioxidants such as α-tocopherol (vitamin E), butylated hydroxyanisole, butylated hydroxytoluene, tertiary butylhydroquinone, lauryl gallate, and β-carotene. The modified FC method reliably quantified ascorbic acid, whereas the conventional method could not. The modified method was reproducible and additive in terms of total antioxidant capacity values of constituents of complex mixtures such as olive oil extract and herbal tea infusion. The trolox equivalent antioxidant capacities of the tested antioxidant compounds correlated well with those found by the Cupric Reducing Antioxidant Capacity reference method.
-
CONJUGATED BLOCK-COPOLYMERS FOR ELECTROLUMINESCENT DIODES
Hilberer, A; Gill, R.E; Herrema, J.K; Malliaras, G.G; Wildeman, J.; Hadziioannou, G
In this article we review results obtained in our laboratory on the design and study of new light-emitting polymers. We are interested in the synthesis and characterisation of block copolymers with regularly alternating conjugated and non conjugated sequences. The blocks giving rise to luminescence
-
Tetrafullerene conjugates for all-organic photovoltaics
Fernández, G.; Sánchez, L.; Veldman, D.; Wienk, M.M.; Atienza, C.M.; Guldi, D.M.; Janssen, R.A.J.; Martin, N.
2008-01-01
The synthesis of two new tetrafullerene nanoconjugates in which four C60 units are covalently connected through different -conjugated oligomers (oligo(p-phenylene ethynylene) and oligo(p-phenylene vinylene)) is described. The photovoltaic (PV) response of these C60-based conjugates was evaluated by
-
Lee, Yiu Yiu; Crauste, Céline; Wang, Hualin; Leung, Ho Hang; Vercauteren, Joseph; Galano, Jean-Marie; Oger, Camille; Durand, Thierry; Wan, Jennifer Man-Fan; Lee, Jetty Chung-Yung
2016-10-17
The effects of extra virgin olive oil (EVOO) and carbon tetrachloride (CCl 4 ) induced oxidative stress in rats were determined by the generation of isoprostanoids. These are known to be robust biomarkers to evaluate nonenzymatic and free radical related oxidation. Other oxidative stress biomarkers such as hydroxyeicosatetraenoic acid products (HETEs) and cholesterol oxidation products (COPs) were also determined. The rodents received a control diet, high-fat diet (20% w/w) composed of extra virgin olive oil (EVOO), corn oil (CO), or lard, and high-fat diets with CCl 4 insult throughout the experimental period. The EVOO diet was found to suppress the formation of isoprostanoids and COPs compared to that of the control. EVOO also had a high total phenolic content and antioxidant activity compared to those of CO and lard and may be contributed to by the hydroxytyrosol component conjugated to fatty acids (HT-FA). This is the first study to identify HT-FA in EVOO, and it was 4-fold higher than that of olive oil, whereas none was found in corn oil. Furthermore, the EVOO diet showed reduced liver lipid vesicles in CCl 4 treated rats compared to that of the control. However, liver toxicity measurements of AST (aspartate transaminase) and ALT (alanine transaminase) activities showed augmentation with CCl 4 treatment but were not alleviated by the diets given. Our findings suggest that EVOO is a daily functional food capable of enhancing the antioxidant system for liver protection; the effect is potentially attributed to the phenolic and lipophenolic (phenol conjugated by fatty acids) content.
-
Novel Aflatoxin Derivatives and Protein Conjugates
Directory of Open Access Journals (Sweden)
Reinhard Niessner
2007-03-01
Full Text Available Aflatoxins, a group of structurally related mycotoxins, are well known for their toxic and carcinogenic effects in humans and animals. Aflatoxin derivatives and protein conjugates are needed for diverse analytical applications. This work describes a reliable and fast synthesis of novel aflatoxin derivatives, purification by preparative HPLC and characterisation by ESI-MS and one- and two-dimensional NMR. Novel aflatoxin bovine serum albumin conjugates were prepared and characterised by UV absorption and MALDI-MS. These aflatoxin protein conjugates are potentially interesting as immunogens for the generation of aflatoxin selective antibodies with novel specificities.
-
Structure Property Relationships in Organic Conjugated Systems
O'Neill, Luke
2008-01-01
A series of pi(п) conjugated oligomers containing 1 to 6 monomer units were studied by absorption and photoluminescence spectroscopies. The results are discussed and examined with regard to the variation of the optical properties with the increase of effective conjugation length. It was found that there was a linear relationship between the positioning of the absorption and photoluminescence maxima plotted against inverse conjugation length. The relationships are in good agreement with the si...
-
Sources and Bioactive Properties of Conjugated Dietary Fatty Acids.
Hennessy, Alan A; Ross, Paul R; Fitzgerald, Gerald F; Stanton, Catherine
2016-04-01
The group of conjugated fatty acids known as conjugated linoleic acid (CLA) isomers have been extensively studied with regard to their bioactive potential in treating some of the most prominent human health malignancies. However, CLA isomers are not the only group of potentially bioactive conjugated fatty acids currently undergoing study. In this regard, isomers of conjugated α-linolenic acid, conjugated nonadecadienoic acid and conjugated eicosapentaenoic acid, to name but a few, have undergone experimental assessment. These studies have indicated many of these conjugated fatty acid isomers commonly possess anti-carcinogenic, anti-adipogenic, anti-inflammatory and immune modulating properties, a number of which will be discussed in this review. The mechanisms through which these bioactivities are mediated have not yet been fully elucidated. However, existing evidence indicates that these fatty acids may play a role in modulating the expression of several oncogenes, cell cycle regulators, and genes associated with energy metabolism. Despite such bioactive potential, interest in these conjugated fatty acids has remained low relative to the CLA isomers. This may be partly attributed to the relatively recent emergence of these fatty acids as bioactives, but also due to a lack of awareness regarding sources from which they can be produced. In this review, we will also highlight the common sources of these conjugated fatty acids, including plants, algae, microbes and chemosynthesis.
-
Preparation and immunological properties of procaine-protein conjugates
International Nuclear Information System (INIS)
Liakopoulou, A.
1981-01-01
Procaine was conjugated to BSA and rat and rabbit Gf using the carbodiimide method and 14 C-procaine as tracer. The composition of the conjugates could be varied depending on the time of incubation and the concentration of procaine in the reaction mixtures. Procaine-BSA conjugates were soluble in water or saline. However, procaine conjugates to rat or rabbit Gf were not readily soluble in saline. These conjugates were good for immunization purposes, but it was cumbersome to work with them when clear solutions were needed, as in the immunochemical procedures used in this study. The immunological properties of the conjugates were studied in rats and rabbits. Rats responded with production of IgGa and precipitating antibodies to the procaine group, but IgE antibodies to the immunogen could not be detected. Furthermore, precipitating antibodies towards the procaine group were raised in rabbits. When BSA was the protein carrier, antibodies to the carrier molecule were also detected in both rats and rabbits. The conjugates of procaine to rat or rabbit Gf did not elicit antibody response to the carrier molecule when used in the homologous species. Hapten inhibition studies suggested that, in the rabbit, antibodies were also produced with specificity directed towards the molecular configuration of the hapten-carrier bond. (author)
-
IRDye78 Conjugates for Near-Infrared Fluorescence Imaging
Directory of Open Access Journals (Sweden)
Atif Zaheer
2002-10-01
Full Text Available The detection of human malignancies by near-infrared (NIR fluorescence will require the conjugation of cancer-specific ligands to NIR fluorophores that have optimal photoproperties and pharmacokinetics. IRDye78, a tetra-sulfonated heptamethine indocyanine NIR fluorophore, meets most of the criteria for an in vivo imaging agent, and is available as an N-hydroxysuccinimide ester for conjugation to low-molecular-weight ligands. However, IRDye78 has a high charge-to-mass ratio, complicating purification of conjugates. It also has a potentially labile linkage between fluorophore and ligand. We have developed an ion-pairing purification strategy for IRDye78 that can be performed with a standard C18 column under neutral conditions, thus preserving the stability of fluorophore, ligand, and conjugate. By employing parallel evaporative light scatter and absorbance detectors, all reactants and products are identified, and conjugate purity is maximized. We describe reversible and irreversible conversions of IRDye78 that can occur during sample purification, and describe methods for preserving conjugate stability. Using seven ligands, spanning several classes of small molecules and peptides (neutral, charged, and/or hydrophobic, we illustrate the robustness of these methods, and confirm that IRDye78 conjugates so purified retain bioactivity and permit NIR fluorescence imaging of specific targets.
-
Preconditioning the modified conjugate gradient method ...
African Journals Online (AJOL)
In this paper, the convergence analysis of the conventional conjugate Gradient method was reviewed. And the convergence analysis of the modified conjugate Gradient method was analysed with our extension on preconditioning the algorithm. Convergence of the algorithm is a function of the condition number of M-1A.
-
Wu, Xianai; Lehmler, Hans-Joachim
2016-02-01
Chiral polychlorinated biphenyl (PCB) congeners, such as PCB 136, are atropselectively metabolized to various hydroxylated PCB metabolites (HO-PCBs). The present study investigates the effect of two thiol antioxidants, glutathione and N-acetyl-cysteine (NAC), on profiles and chiral signatures of PCB 136 and its HO-PCB metabolites in rat liver microsomal incubations. Liver microsomes prepared from rats pretreated with phenobarbital were incubated with PCB 136 (5 μM) in the presence of the respective antioxidant (0-10 mM), and levels and chiral signatures of PCB 136 and its HO-PCB metabolites were determined. Three metabolites, 5-136 (2,2',3,3',6,6'-hexachlorobiphenyl-5-ol), 4-136 (2,2',3,3',6,6'-hexachlorobiphenyl-4-ol), and 4,5-136 (2,2',3,3',6,6'-hexachlorobiphenyl-4,5-diol), were detected in all incubations, with 5-136 being the major metabolite. Compared to microsomal incubations without antioxidant, levels of 4,5-136 increased with increasing antioxidant concentration, whereas levels of PCB 136 and both mono-HO-PCBs were not affected by the presence of either antioxidant. PCB 136, 4-136, and 5-136 displayed significant atropisomeric enrichment; however, the direction and extent of the atropisomeric enrichment was not altered in the presence of an antioxidant. Because 4,5-136 can either be conjugated to a sulfate or glucuronide metabolite that is readily excreted or further oxidized a potentially toxic PCB 136 quinone, the effect of both thiol antioxidants on 4,5-136 formation suggests that disruptions of glutathione homeostasis may alter the balance between both metabolic pathways and, thus, PCB 136 toxicity in vivo.
-
Cao, Na; Feng, Si-Shen
2008-10-01
To develop a polymer-anticancer drug conjugate, D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) was employed as a carrier of doxorubicin (DOX) to enhance its therapeutic effects and reduce its side effects. Doxorubicin was chemically conjugated to TPGS. The molecular structure, drug loading efficiency, drug release kinetics and stability of the conjugate were characterized. The cellular uptake, intracellular distribution, and cytotoxicity were accessed by using MCF-7 breast cancer cells and C6 glioma cells as in vitro cell model. The conjugate showed higher cellular uptake efficiency and broader distribution within the cells. Judged by IC(50), the conjugate was found 31.8, 69.6, 84.1% more effective with MCF-7 cells and 43.9, 87.7, 42.2% more effective with C6 cells than the parent drug after 24, 48, 72 h culture, respectively. The in vivo pharmacokinetics and biodistribution were investigated after an i.v. administration at 5 mg DOX/kg body weight in rats. Promisingly, 4.5-fold increase in the half-life and 24-fold increase in the area-under-the-curve (AUC) of DOX were achieved for the TPGS-DOX conjugate compared with the free DOX. The drug level in heart, gastric and intestine was significantly reduced, which is an indication of reduced side effects. Our TPGS-DOX conjugate showed great potential to be a prodrug of higher therapeutic effects and fewer side effects than DOX itself.
-
International Nuclear Information System (INIS)
Borshchevs'ka, M.Yi.; Popov, V.V.; Abramova, L.P.; Kuz'myinova, Yi.A.
1995-01-01
The authors have studied the influence of physiologically active complex isolated from human amnion on the state of lipid peroxide oxidation according to diene conjugate and malonic dialdehyde amount and antioxidant enzyme activity (catalase and glutationperoxidase) in the blood of the rats exposed to single total irradiation in different doses (4 and 6 Gy) was studied. Definite changes of peroxide process intensity and reduction of the enzymes activity were shown to be observed in the blood of experimental animals even at long terms after the radiation exposure. Under the background of radiation exposure, administration of physiologically active complex isolated from human amnion produced protective effect on antioxidant enzyme activity which promoted normalization of peroxidation processes within the post-radiation period
-
Bis-polymer lipid-peptide conjugates and nanoparticles thereof
Energy Technology Data Exchange (ETDEWEB)
Xu, Ting; Dong, He; Shu, Jessica; Dube, Nikhil
2018-04-24
The present invention provides bis-polymer lipid-peptide conjugates containing a hydrophobic block and headgroup containing a helical peptide and two polymer blocks. The conjugates can self-assemble to form helix bundle subunits, which in turn assemble to provide micellar nanocarriers for drug cargos and other agents. Particles containing the conjugates and methods for forming the particles are also disclosed.
-
Soluble antioxidant compounds regenerate the antioxidants bound to insoluble parts of foods.
Çelik, Ecem Evrim; Gökmen, Vural; Fogliano, Vincenzo
2013-10-30
This study aimed to investigate the regeneration potential of antioxidant capacity of an insoluble food matrix. Investigations were performed in vitro with several food matrices rich in dietary fiber (DF) and bound antioxidants. After removal of the soluble fraction, the antioxidant capacity (AC) of the insoluble fraction was measured by the QUENCHER procedure using ABTS(•+) or DPPH(•) radicals. After measurement, the insoluble residue was washed out to remove the excess of radicals and treated with pure antioxidant solution or antioxidant-rich beverage to regenerate depleted antioxidants on the fiber. Results revealed that the antioxidant capacity of compounds chemically bound to the insoluble moiety could be reconstituted in the presence of other hydrogen-donating substances in the liquid phase. Regeneration efficiency was found to range between 21.5 and 154.3% depending on the type of insoluble food matrix and regeneration agent. Among the food matrices studied, cereal products were found to have slightly higher regeneration efficiency, whereas antioxidant-rich beverages were more effective than pure antioxidants as regeneration agents. Taking wheat bran as reference insoluble material, the regeneration abilities of beverages were in the following order: green tea > espresso coffee > black tea > instant coffee > orange juice > red wine. These results highlighted the possible physiological relevance of antioxidants bound to the insoluble food material in the gastrointestinal tract. During the digestion process they could react with the free radicals and at the same time they can be regenerated by other soluble antioxidant compounds present in the meal.
-
Conjugated polymer nanoparticles, methods of using, and methods of making
Habuchi, Satoshi; Piwonski, Hubert Marek; Michinobu, Tsuyoshi
2017-01-01
Embodiments of the present disclosure provide for conjugated polymer nanoparticle, method of making conjugated polymer nanoparticles, method of using conjugated polymer nanoparticle, polymers, and the like.
-
Conjugated polymer nanoparticles, methods of using, and methods of making
Habuchi, Satoshi
2017-03-16
Embodiments of the present disclosure provide for conjugated polymer nanoparticle, method of making conjugated polymer nanoparticles, method of using conjugated polymer nanoparticle, polymers, and the like.
-
Dietary antioxidants and exercise.
Powers, Scott K; DeRuisseau, Keith C; Quindry, John; Hamilton, Karyn L
2004-01-01
Muscular exercise promotes the production of radicals and other reactive oxygen species in the working muscle. Growing evidence indicates that reactive oxygen species are responsible for exercise-induced protein oxidation and contribute to muscle fatigue. To protect against exercise-induced oxidative injury, muscle cells contain complex endogenous cellular defence mechanisms (enzymatic and non-enzymatic antioxidants) to eliminate reactive oxygen species. Furthermore, exogenous dietary antioxidants interact with endogenous antioxidants to form a cooperative network of cellular antioxidants. Knowledge that exercise-induced oxidant formation can contribute to muscle fatigue has resulted in numerous investigations examining the effects of antioxidant supplementation on human exercise performance. To date, there is limited evidence that dietary supplementation with antioxidants will improve human performance. Furthermore, it is currently unclear whether regular vigorous exercise increases the need for dietary intake of antioxidants. Clearly, additional research that analyses the antioxidant requirements of individual athletes is needed.
-
Nonlinear conjugate gradient methods in micromagnetics
Directory of Open Access Journals (Sweden)
J. Fischbacher
2017-04-01
Full Text Available Conjugate gradient methods for energy minimization in micromagnetics are compared. The comparison of analytic results with numerical simulation shows that standard conjugate gradient method may fail to produce correct results. A method that restricts the step length in the line search is introduced, in order to avoid this problem. When the step length in the line search is controlled, conjugate gradient techniques are a fast and reliable way to compute the hysteresis properties of permanent magnets. The method is applied to investigate demagnetizing effects in NdFe12 based permanent magnets. The reduction of the coercive field by demagnetizing effects is μ0ΔH = 1.4 T at 450 K.
-
Usta, M.; Wortelboer, H.M.; Vervoort, J.J.M.; Boersma, M.G.; Rietjens, I.M.C.M.; Bladeren, van P.J.; Cnubben, N.H.P.
2007-01-01
Curcumin, an alpha,beta-unsaturated carbonyl compound, reacts with glutathione, leading to the formation of two monoglutathionyl curcumin conjugates. In the present study, the structures of both glutathione conjugates of curcumin were identified by LC-MS and one- and two-dimensional H-1 NMR
-
Selective Covalent Conjugation of Phosphorothioate DNA Oligonucleotides with Streptavidin
Directory of Open Access Journals (Sweden)
Christof M. Niemeyer
2011-08-01
Full Text Available Protein-DNA conjugates have found numerous applications in the field of diagnostics and nanobiotechnology, however, their intrinsic susceptibility to DNA degradation by nucleases represents a major obstacle for many applications. We here report the selective covalent conjugation of the protein streptavidin (STV with phosphorothioate oligonucleotides (psDNA containing a terminal alkylthiolgroup as the chemically addressable linking unit, using a heterobifunctional NHS-/maleimide crosslinker. The psDNA-STV conjugates were synthesized in about 10% isolated yields. We demonstrate that the terminal alkylthiol group selectively reacts with the maleimide while the backbone sulfur atoms are not engaged in chemical conjugation. The novel psDNA-STV conjugates retain their binding capabilities for both biotinylated macromolecules and the complementary nucleic acid. Moreover, the psDNA-STV conjugate retained its binding capacity for complementary oligomers even after a nuclease digestion step, which effectively degrades deoxyribonucleotide oligomers and thus the binding capability of regular DNA-STV conjugates. The psDNA-STV therefore hold particular promise for applications e.g. in proteome research and novel biosensing devices, where interfering endogenous nucleic acids need to be removed from analytes by nuclease digestion.
-
DEFF Research Database (Denmark)
Nielsen, K.T.; Spanggaard, H.; Krebs, Frederik C
2004-01-01
. Electroluminescent devices of the homopolymer itself and of the zinc-porphyrin containing polymer were prepared and the nature of the electroluminescence was characterized. The homopolymer segments were found to optically pump the emission of the zinc-porphyrin dye moities. The homopolymer exhibits blue......Zinc-porphyrin dye molecules were incorporated into the backbone of a conjugated polymer material by a method, which allowed for the incorporation of only one zinc-porphyrin dye molecule into the backbone of each conjugated polymer molecule. The electronic properties of the homopolymer were...
-
Lipid-peptide-polymer conjugates and nanoparticles thereof
Xu, Ting; Dong, He; Shu, Jessica
2015-06-02
The present invention provides a conjugate having a peptide with from about 10 to about 100 amino acids, wherein the peptide adopts a helical structure. The conjugate also includes a first polymer covalently linked to the peptide, and a hydrophobic moiety covalently linked to the N-terminus of the peptide, wherein the hydrophobic moiety comprises a second polymer or a lipid moiety. The present invention also provides helix bundles form by self-assembling the conjugates, and particles formed by self-assembling the helix bundles. Methods of preparing the helix bundles and particles are also provided.
-
Novel β-cyclodextrin-eosin conjugates.
Benkovics, Gábor; Afonso, Damien; Darcsi, András; Béni, Szabolcs; Conoci, Sabrina; Fenyvesi, Éva; Szente, Lajos; Malanga, Milo; Sortino, Salvatore
2017-01-01
Eosin B (EoB) and eosin Y (EoY), two xanthene dye derivatives with photosensitizing ability were prepared in high purity through an improved synthetic route. The dyes were grafted to a 6-monoamino-β-cyclodextrin scaffold under mild reaction conditions through a stable amide linkage using the coupling agent 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride. The molecular conjugates, well soluble in aqueous medium, were extensively characterized by 1D and 2D NMR spectroscopy and mass spectrometry. Preliminary spectroscopic investigations showed that the β-cyclodextrin-EoY conjugate retains both the fluorescence properties and the capability to photogenerate singlet oxygen of the unbound chromophore. In contrast, the corresponding β-cyclodextrin-EoB conjugate did not show either relevant emission or photosensitizing activity probably due to aggregation in aqueous medium, which precludes any response to light excitation.
-
Novel β-cyclodextrin–eosin conjugates
Directory of Open Access Journals (Sweden)
Gábor Benkovics
2017-03-01
Full Text Available Eosin B (EoB and eosin Y (EoY, two xanthene dye derivatives with photosensitizing ability were prepared in high purity through an improved synthetic route. The dyes were grafted to a 6-monoamino-β-cyclodextrin scaffold under mild reaction conditions through a stable amide linkage using the coupling agent 4-(4,6-dimethoxy-1,3,5-triazin-2-yl-4-methylmorpholinium chloride. The molecular conjugates, well soluble in aqueous medium, were extensively characterized by 1D and 2D NMR spectroscopy and mass spectrometry. Preliminary spectroscopic investigations showed that the β-cyclodextrin–EoY conjugate retains both the fluorescence properties and the capability to photogenerate singlet oxygen of the unbound chromophore. In contrast, the corresponding β-cyclodextrin–EoB conjugate did not show either relevant emission or photosensitizing activity probably due to aggregation in aqueous medium, which precludes any response to light excitation.
-
Directory of Open Access Journals (Sweden)
Hongqin Jiang
2015-07-01
Full Text Available Lycopene, a red non-provitamin A carotenoid, mainly presenting in tomato and tomato byproducts, has the highest antioxidant activity among carotenoids because of its high number of conjugated double bonds. The objective of this study was to investigate the effect of lycopene supplementation in the diet on plasma lipid profile, lipid peroxidation and antioxidant defense system in feedlot lamb. Twenty-eight Bamei male lambs (90 days old were divided into four groups and fed a basal diet (LP0, 40:60 roughage: concentrate or the basal diet supplemented with 50, 100, and 200 mg/kg lycopene. After 120 days of feeding, all lambs were slaughtered and sampled. Dietary lycopene supplementation significantly reduced the levels of plasma total cholesterol (p0.05. The levels of TG (p<0.001 and LDL-C (p<0.001 were decreased with the feeding time extension, and both showed a linear trend (p<0.01. Malondialdehyde level in plasma and liver decreased linearly with the increase of lycopene inclusion levels (p<0.01. Dietary lycopene intake linearly increased the plasma antioxidant vitamin E level (p<0.001, total antioxidant capacity (T-AOC, p<0.05, and activities of catalase (CAT, p<0.01, glutathione peroxidase (GSH-Px, p<0.05 and superoxide dismutase (SOD, p<0.05. The plasma T-AOC and activities of GSH-Px and SOD decreased with the extension of the feeding time. In liver, dietary lycopene inclusion showed similar antioxidant effects with respect to activities of CAT (p<0.05, linearly and SOD (p<0.001, linearly. Therefore, it was concluded that lycopene supplementation improved the antioxidant status of the lamb and optimized the plasma lipid profile, the dosage of 200 mg lycopene/kg feed might be desirable for growing lambs to prevent environment stress and maintain normal physiological metabolism.
-
Photodynamic tissue adhesion with chlorin(e6) protein conjugates.
Khadem, J; Veloso, A A; Tolentino, F; Hasan, T; Hamblin, M R
1999-12-01
To test the hypothesis that a photodynamic laser-activated tissue solder would perform better in sealing scleral incisions when the photosensitizer was covalently linked to the protein than when it was noncovalently mixed. Conjugates and mixtures were prepared between the photosensitizer chlorin(e6) and various proteins (albumin, fibrinogen, and gelatin) in different ratios and used to weld penetrating scleral incisions made in human cadaveric eyes. A blue-green (488-514 nm) argon laser activated the adhesive, and the strength of the closure was measured by increasing the intraocular pressure until the wound showed leakage. Both covalent conjugates and noncovalent mixtures showed a light dose-dependent increase in leaking pressure. A preparation of albumin chlorin(e6) conjugate with additional albumin added (2.5 protein to chlorin(e6) molar ratio) showed significantly higher weld strength than other protein conjugates and mixtures. This is the first report of dye-protein conjugates as tissue solders. These conjugates may have applications in ophthalmology.
-
Usta, M.; Wortelboer, H.M.; Vervoort, J.; Boersma, M.G.; Rietjens, I.M.C.M.; Bladeren, P.J. van; Cnubben, N.H.P.
2007-01-01
Curcumin, an α,β-unsaturated carbonyl compound, reacts with glutathione, leading to the formation of two monoglutathionyl curcumin conjugates. In the present study, the structures of both glutathione conjugates of curcumin were identified by LC-MS and one- and two-dimensional 1H NMR analysis, and
-
DENDRIMER CONJUGATES FOR SELECTIVE OF PROTEIN AGGREGATES
DEFF Research Database (Denmark)
2004-01-01
Dendrimer conjugates are presented, which are formed between a dendrimer and a protein solubilising substance. Such dendrimer conjugates are effective in the treatment of protein aggregate-related diseases (e.g. prion-related diseases). The protein solubilising substance and the dendrimer together...
-
Directory of Open Access Journals (Sweden)
Jacob Thomas
Full Text Available Integrative and conjugative elements (ICEs are agents of horizontal gene transfer and have major roles in evolution and acquisition of new traits, including antibiotic resistances. ICEs are found integrated in a host chromosome and can excise and transfer to recipient bacteria via conjugation. Conjugation involves nicking of the ICE origin of transfer (oriT by the ICE-encoded relaxase and transfer of the nicked single strand of ICE DNA. For ICEBs1 of Bacillus subtilis, nicking of oriT by the ICEBs1 relaxase NicK also initiates rolling circle replication. This autonomous replication of ICEBs1 is critical for stability of the excised element in growing cells. We found a conserved and previously uncharacterized ICE gene that is required for conjugation and replication of ICEBs1. Our results indicate that this gene, helP (formerly ydcP, encodes a helicase processivity factor that enables the host-encoded helicase PcrA to unwind the double-stranded ICEBs1 DNA. HelP was required for both conjugation and replication of ICEBs1, and HelP and NicK were the only ICEBs1 proteins needed for replication from ICEBs1 oriT. Using chromatin immunoprecipitation, we measured association of HelP, NicK, PcrA, and the host-encoded single-strand DNA binding protein Ssb with ICEBs1. We found that NicK was required for association of HelP and PcrA with ICEBs1 DNA. HelP was required for association of PcrA and Ssb with ICEBs1 regions distal, but not proximal, to oriT, indicating that PcrA needs HelP to progress beyond nicked oriT and unwind ICEBs1. In vitro, HelP directly stimulated the helicase activity of the PcrA homologue UvrD. Our findings demonstrate that HelP is a helicase processivity factor needed for efficient unwinding of ICEBs1 for conjugation and replication. Homologues of HelP and PcrA-type helicases are encoded on many known and putative ICEs. We propose that these factors are essential for ICE conjugation, replication, and genetic stability.
-
Antioxidants of Edible Mushrooms
Directory of Open Access Journals (Sweden)
Maja Kozarski
2015-10-01
Full Text Available Oxidative stress caused by an imbalanced metabolism and an excess of reactive oxygen species (ROS lead to a range of health disorders in humans. Our endogenous antioxidant defense mechanisms and our dietary intake of antioxidants potentially regulate our oxidative homeostasis. Numerous synthetic antioxidants can effectively improve defense mechanisms, but because of their adverse toxic effects under certain conditions, preference is given to natural compounds. Consequently, the requirements for natural, alternative sources of antioxidant foods identified in edible mushrooms, as well as the mechanistic action involved in their antioxidant properties, have increased rapidly. Chemical composition and antioxidant potential of mushrooms have been intensively studied. Edible mushrooms might be used directly in enhancement of antioxidant defenses through dietary supplementation to reduce the level of oxidative stress. Wild or cultivated, they have been related to significant antioxidant properties due to their bioactive compounds, such as polyphenols, polysaccharides, vitamins, carotenoids and minerals. Antioxidant and health benefits, observed in edible mushrooms, seem an additional reason for their traditional use as a popular delicacy food. This review discusses the consumption of edible mushrooms as a powerful instrument in maintaining health, longevity and life quality.
-
Zago, Erika; Durand, Erwann; Barouh, Nathalie; Lecomte, Jérôme; Villeneuve, Pierre; Aouf, Chahinez
2015-10-21
4-Vinyl guaiacol (2) was lipophilized through the electrophilic addition of peracids to its vinylic double bond. Those peracids were formed in situ, by the Candida antarctica lipase-B-assisted perhydrolysis of carboxylic acids ranging from C2 to C18, in hydrogen peroxide solution. The addition of peracids with 4-8 carbons in their alkyl chains led to the formation of two regioisomers, with the prevalence of hydroxyesters bearing a primary free hydroxyl (4c-4e). This prevalence became more pronounced when peracids with longer alkyl chains (C10-C18) were used. In this case, only isomers 4f-4h were formed. The antioxidant activity of the resulting hydroxyesters was assessed by means of the conjugated autoxidizable triene (CAT) assay, and it was found out that the 4-vinyl guaiacol antioxidant activity was significantly increased by grafting alkyl chains with 2-8 carbons.
-
... version Home Brain, Spinal Cord, and Nerve Disorders Cranial Nerve Disorders Conjugate Gaze Palsies Horizontal gaze palsy Vertical ... Version. DOCTORS: Click here for the Professional Version Cranial Nerve Disorders Overview of the Cranial Nerves Internuclear Ophthalmoplegia ...
-
Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways.
Corrochano, Alberto R; Buckin, Vitaly; Kelly, Phil M; Giblin, Linda
2018-03-28
Oxidative stress contributes to cell injury and aggravates several chronic diseases. Dietary antioxidants help the body to fight against free radicals and, therefore, avoid or reduce oxidative stress. Recently, proteins from milk whey liquid have been described as antioxidants. This review summarizes the evidence that whey products exhibit radical scavenging activity and reducing power. It examines the processing and treatment attempts to increase the antioxidant bioactivity and identifies 1 enzyme, subtilisin, which consistently produces the most potent whey fractions. The review compares whey from different milk sources and puts whey proteins in the context of other known food antioxidants. However, for efficacy, the antioxidant activity of whey proteins must not only survive processing, but also upper gut transit and arrival in the bloodstream, if whey products are to promote antioxidant levels in target organs. Studies reveal that direct cell exposure to whey samples increases intracellular antioxidants such as glutathione. However, the physiological relevance of these in vitro assays is questionable, and evidence is conflicting from dietary intervention trials, with both rats and humans, that whey products can boost cellular antioxidant biomarkers. Copyright © 2018 American Dairy Science Association. Published by Elsevier Inc. All rights reserved.
-
Synthesis of Mikto-Arm Star Peptide Conjugates.
Koo, Jin Mo; Su, Hao; Lin, Yi-An; Cui, Honggang
2018-01-01
Mikto-arm star peptide conjugates are an emerging class of self-assembling peptide-based structural units that contain three or more auxiliary segments of different chemical compositions and/or functionalities. This group of molecules exhibit interesting self-assembly behavior in solution due to their chemically asymmetric topology. Here we describe the detailed procedure for synthesis of an ABC Mikto-arm star peptide conjugate in which two immiscible entities (a saturated hydrocarbon and a hydrophobic and lipophobic fluorocarbon) are conjugated onto a short β-sheet forming peptide sequence, GNNQQNY, derived from the Sup35 prion, through a lysine junction. Automated and manual Fmoc-solid phase synthesis techniques are used to synthesize the Mikto-arm star peptide conjugates, followed by HPLC purification. We envision that this set of protocols can afford a versatile platform to synthesize a new class of peptidic building units for diverse applications.
-
Gold Nanoparticle Conjugation Enhances the Antiacanthamoebic Effects of Chlorhexidine
Aqeel, Yousuf; Siddiqui, Ruqaiyyah; Anwar, Ayaz; Shah, Muhammad Raza
2015-01-01
Acanthamoeba keratitis is a serious infection with blinding consequences and often associated with contact lens wear. Early diagnosis, followed by aggressive topical application of drugs, is a prerequisite in successful treatment, but even then prognosis remains poor. Several drugs have shown promise, including chlorhexidine gluconate; however, host cell toxicity at physiologically relevant concentrations remains a challenge. Nanoparticles, subcolloidal structures ranging in size from 10 to 100 nm, are effective drug carriers for enhancing drug potency. The overall aim of the present study was to determine whether conjugation with gold nanoparticles enhances the antiacanthamoebic potential of chlorhexidine. Gold-conjugated chlorhexidine nanoparticles were synthesized. Briefly, gold solution was mixed with chlorhexidine and reduced by adding sodium borohydride, resulting in an intense deep red color, indicative of colloidal gold-conjugated chlorhexidine nanoparticles. The synthesis was confirmed using UV-visible spectrophotometry that shows a plasmon resonance peak of 500 to 550 nm, indicative of gold nanoparticles. Further characterization using matrix-assisted laser desorption ionization-mass spectrometry showed a gold-conjugated chlorhexidine complex at m/z 699 ranging in size from 20 to 100 nm, as determined using atomic force microscopy. To determine the amoebicidal and amoebistatic effects, amoebae were incubated with gold-conjugated chlorhexidine nanoparticles. For controls, amoebae also were incubated with gold and silver nanoparticles alone, chlorhexidine alone, neomycin-conjugated nanoparticles, and neomycin alone. The findings showed that gold-conjugated chlorhexidine nanoparticles exhibited significant amoebicidal and amoebistatic effects at 5 μM. Amoebicidal effects were observed by parasite viability testing using a Trypan blue exclusion assay and flow-cytometric analysis using propidium iodide, while amoebistatic effects were observed using growth
-
Analytical characterization of polymer-drug conjugates
International Nuclear Information System (INIS)
Rizzo, V.; Gigli, M.; Pinciroli, V.
1998-01-01
A few polymeric conjugates of antitumor drugs have been recently developed in view of possible therapeutic advantages: solubilization of sparingly soluble drugs in water, improvement of therapeutic index, organ targeting through a second chemical species bound to the same polymeric chain. In this article it's described the analytical approach used in the characterization of the conjugates for chemical identity, purity and strength of the contained active ingredient. The techniques are: high field NMR and size exclusion chromatography with non-aqueous mobile phase for identity; selective hydrolysis and HPLC for strength and purity. A complete and reliable picture is thus obtained both for qualitative and for quantitative aspects. This is an important step forward in the direction of further development and marketing of polymer-drug conjugates [it
-
Conjugate gradient algorithms using multiple recursions
Energy Technology Data Exchange (ETDEWEB)
Barth, T.; Manteuffel, T.
1996-12-31
Much is already known about when a conjugate gradient method can be implemented with short recursions for the direction vectors. The work done in 1984 by Faber and Manteuffel gave necessary and sufficient conditions on the iteration matrix A, in order for a conjugate gradient method to be implemented with a single recursion of a certain form. However, this form does not take into account all possible recursions. This became evident when Jagels and Reichel used an algorithm of Gragg for unitary matrices to demonstrate that the class of matrices for which a practical conjugate gradient algorithm exists can be extended to include unitary and shifted unitary matrices. The implementation uses short double recursions for the direction vectors. This motivates the study of multiple recursion algorithms.
-
Four-wave mixing and phase conjugation in plasmas
International Nuclear Information System (INIS)
Federici, J.F.
1989-01-01
Nonlinear optical effects such as Stimulated Brillouin Scattering, Stimulated Raman Scattering, self-focusing, wave-mixing, parametric mixing, etc., have a long history in plasma physics. Recently, four-wave mixing in plasmas and its applications to phase conjugation has been extensively studied. Although four-wave mixing (FWM), using various nonlinear mediums, has many practical applications in the visible regime, no successful attempt has been made to study or demonstrate FWM for wavelengths longer than 10μm. Plasmas as phase conjugate mirrors have received considerable attention since they become more efficient at longer wavelengths (far-infrared to microwave). The purpose of this thesis is to study various fundamental issues which concern the suitability of plasmas for four-wave mixing and phase conjugation. The major contributions of this thesis are the identification and study of thermal and ionization nonlinearities as potential four-wave mixing and phase conjugation mechanisms and the study of the affect of density inhomogeneities on the FWM process. Using a fluid description for the plasma, this thesis demonstrates that collisional heating generates a thermal force which substantially enhances the phase conjugate reflectivity. The prospect of using a novel ionization nonlinearity in weakly ionized plasmas for wave-mixing and phase conjugation is discussed. The ionization nonlinearity arises from localized heating of the plasma by the beat-wave. Wherever, the local temperature is increased, a plasma density grating is produced due to increased electron-impact ionization. Numerical estimates of the phase conjugate reflectivity indicate reflectivities in the range of 10 -4 -10 -3 are possible in a weakly ionized steady-state gas discharge plasma
-
Hu, Shun; Yu, Weili; Hu, Chunyang; Wei, Dong; Shen, Lijuan; Hu, Tao; Yi, Youjin
2017-11-01
Mycobacterium tuberculosis (Mtb) is a serious fatal pathogen that causes tuberculosis (TB). Effective vaccination is urgently needed to deal with the serious threat from TB. Mtb-secreted protein antigens are important virulence determinants of Mtb with poor immunogenicity. Adjuvants and antigen delivery systems are thus highly desired to improve the immunogenicity of protein antigens. Inulin is a biocompatible polysaccharide (PS) adjuvant that can stimulate a strong cellular and humoral immunity. Bacterial capsular PS and haptens have been conjugated with cross-reacting material 197 (CRM 197 ) to improve their immunogenicity. CFP10 and TB10.4 were two Mtb-secreted immunodominant protein antigens. A CFP10-TB10.4 fusion protein (CT) was used as the antigen for covalent conjugation with the CRM 197 -inulin conjugate (CRM-inu). The resultant conjugate (CT-CRM-inu) elicited high CT-specific IgG titers, stimulated splenocyte proliferation and provoked the secretion of Th1-type and Th2-type cytokines. Conjugation with CRM-inu significantly prolonged the systemic circulation of CT and exposure to the immune system. Moreover, CT-CRM-inu showed no apparent toxicity to cardiac, hepatic and renal functions. Thus, conjugation of CT with CRM-inu provided an effective strategy for development of protein-based vaccines against Mtb infection. Copyright © 2017 Elsevier Ltd. All rights reserved.
-
O:2-CRM(197) conjugates against Salmonella Paratyphi A.
Micoli, Francesca; Rondini, Simona; Gavini, Massimiliano; Lanzilao, Luisa; Medaglini, Donata; Saul, Allan; Martin, Laura B
2012-01-01
Enteric fevers remain a common and serious disease, affecting mainly children and adolescents in developing countries. Salmonella enterica serovar Typhi was believed to cause most enteric fever episodes, but several recent reports have shown an increasing incidence of S. Paratyphi A, encouraging the development of a bivalent vaccine to protect against both serovars, especially considering that at present there is no vaccine against S. Paratyphi A. The O-specific polysaccharide (O:2) of S. Paratyphi A is a protective antigen and clinical data have previously demonstrated the potential of using O:2 conjugate vaccines. Here we describe a new conjugation chemistry to link O:2 and the carrier protein CRM(197), using the terminus 3-deoxy-D-manno-octulosonic acid (KDO), thus leaving the O:2 chain unmodified. The new conjugates were tested in mice and compared with other O:2-antigen conjugates, synthesized adopting previously described methods that use CRM(197) as carrier protein. The newly developed conjugation chemistry yielded immunogenic conjugates with strong serum bactericidal activity against S. Paratyphi A.
-
O:2-CRM(197 conjugates against Salmonella Paratyphi A.
Directory of Open Access Journals (Sweden)
Francesca Micoli
Full Text Available Enteric fevers remain a common and serious disease, affecting mainly children and adolescents in developing countries. Salmonella enterica serovar Typhi was believed to cause most enteric fever episodes, but several recent reports have shown an increasing incidence of S. Paratyphi A, encouraging the development of a bivalent vaccine to protect against both serovars, especially considering that at present there is no vaccine against S. Paratyphi A. The O-specific polysaccharide (O:2 of S. Paratyphi A is a protective antigen and clinical data have previously demonstrated the potential of using O:2 conjugate vaccines. Here we describe a new conjugation chemistry to link O:2 and the carrier protein CRM(197, using the terminus 3-deoxy-D-manno-octulosonic acid (KDO, thus leaving the O:2 chain unmodified. The new conjugates were tested in mice and compared with other O:2-antigen conjugates, synthesized adopting previously described methods that use CRM(197 as carrier protein. The newly developed conjugation chemistry yielded immunogenic conjugates with strong serum bactericidal activity against S. Paratyphi A.
-
Czech Academy of Sciences Publication Activity Database
Etrych, Tomáš; Tsukigawa, K.; Nakamura, H.; Chytil, Petr; Fang, J.; Ulbrich, Karel; Otagiri, M.; Maeda, H.
2017-01-01
Roč. 106, 30 August (2017), s. 10-19 ISSN 0928-0987 R&D Projects: GA ČR(CZ) GA15-02986S; GA MŠk(CZ) LQ1604; GA MŠk(CZ) ED1.1.00/02.0109 Grant - others:AV ČR,Japan Society for the Promotion of Science(CZ) JSPS-16-05 Program:Bilaterální spolupráce Institutional support: RVO:61389013 Keywords : pirarubicin * PHPMA conjugate * tandem-diblock PHPMA conjugate Subject RIV: FR - Pharmacology ; Medidal Chemistry OBOR OECD: Pharmacology and pharmacy Impact factor: 3.756, year: 2016
-
Integrated circuits based on conjugated polymer monolayer.
Li, Mengmeng; Mangalore, Deepthi Kamath; Zhao, Jingbo; Carpenter, Joshua H; Yan, Hongping; Ade, Harald; Yan, He; Müllen, Klaus; Blom, Paul W M; Pisula, Wojciech; de Leeuw, Dago M; Asadi, Kamal
2018-01-31
It is still a great challenge to fabricate conjugated polymer monolayer field-effect transistors (PoM-FETs) due to intricate crystallization and film formation of conjugated polymers. Here we demonstrate PoM-FETs based on a single monolayer of a conjugated polymer. The resulting PoM-FETs are highly reproducible and exhibit charge carrier mobilities reaching 3 cm 2 V -1 s -1 . The high performance is attributed to the strong interactions of the polymer chains present already in solution leading to pronounced edge-on packing and well-defined microstructure in the monolayer. The high reproducibility enables the integration of discrete unipolar PoM-FETs into inverters and ring oscillators. Real logic functionality has been demonstrated by constructing a 15-bit code generator in which hundreds of self-assembled PoM-FETs are addressed simultaneously. Our results provide the state-of-the-art example of integrated circuits based on a conjugated polymer monolayer, opening prospective pathways for bottom-up organic electronics.
-
METHOD OF CONJUGATED CIRCULAR ARCS TRACING
Directory of Open Access Journals (Sweden)
N. Ageyev Vladimir
2017-01-01
Full Text Available The geometric properties of conjugated circular arcs connecting two points on the plane with set directions of tan- gent vectors are studied in the work. It is shown that pairs of conjugated circular arcs with the same conditions in frontier points create one-parameter set of smooth curves tightly filling all the plane. One of the basic properties of this set is the fact that all coupling points of circular arcs are on the circular curve going through the initially given points. The circle radius depends on the direction of tangent vectors. Any point of the circle curve, named auxiliary in this work, determines a pair of conjugated arcs with given boundary conditions. One more condition of the auxiliary circle curve is that it divides the plane into two parts. The arcs going from the initial point are out of the circle limited by this circle curve and the arcs coming to the final point are inside it. These properties are the basis for the method of conjugated circular arcs tracing pro- posed in this article. The algorithm is rather simple and allows to fulfill all the needed plottings using only the divider and ruler. Two concrete examples are considered. The first one is related to the problem of tracing of a pair of conjugated arcs with the minimal curve jump when going through the coupling point. The second one demonstrates the possibility of trac- ing of the smooth curve going through any three points on the plane under condition that in the initial and final points the directions of tangent vectors are given. The proposed methods of conjugated circular arcs tracing can be applied in solving of a wide variety of problems connected with the tracing of cam contours, for example pattern curves in textile industry or in computer-aided-design systems when programming of looms with numeric control.
-
Frank, Thomas; Netzel, Gabriele; Kammerer, Dietmar R; Carle, Reinhold; Kler, Adolf; Kriesl, Erwin; Bitsch, Irmgard; Bitsch, Roland; Netzel, Michael
2012-08-15
To evaluate health benefits attributed to Hibiscus sabdariffa L. a randomized, open-label, two-way crossover study was undertaken to compare the impact of an aqueous H. sabdariffa L. extract (HSE) on the systemic antioxidant potential (AOP; assayed by ferric reducing antioxidant power (FRAP)) with a reference treatment (water) in eight healthy volunteers. The biokinetic variables were the areas under the curve (AUC) of plasma FRAP, ascorbic acid and urate that are above the pre-dose concentration, and the amounts excreted into urine within 24 h (Ae(0-24) ) of antioxidants as assayed by FRAP, ascorbic acid, uric acid, malondialdehyde (biomarker for oxidative stress), and hippuric acid (metabolite and potential biomarker for total polyphenol intake). HSE caused significantly higher plasma AUC of FRAP, an increase in Ae(0-24) of FRAP, ascorbic acid and hippuric acid, whereas malondialdehyde excretion was reduced. Furthermore, the main hibiscus anthocyanins as well as one glucuronide conjugate could be quantified in the volunteers' urine (0.02% of the administered dose). The aqueous HSE investigated in this study enhanced the systemic AOP and reduced the oxidative stress in humans. Furthermore, the increased urinary hippuric acid excretion after HSE consumption indicates a high biotransformation of the ingested HSE polyphenols, most likely caused by the colonic microbiota. Copyright © 2012 Society of Chemical Industry.
-
DEFF Research Database (Denmark)
Hansen, Paul Robert
2015-01-01
To produce antibodies against synthetic peptides it is necessary to couple them to a protein carrier. This chapter provides a nonspecialist overview of peptide-carrier conjugation. Furthermore, a protocol for coupling cysteine-containing peptides to bovine serum albumin is outlined....
-
Class, Kinship Density, and Conjugal Role Segregation.
Hill, Malcolm D.
1988-01-01
Studied conjugal role segregation in 150 married women from intact families in working-class community. Found that, although involvement in dense kinship networks was associated with conjugal role segregation, respondents' attitudes toward marital roles and phase of family cycle when young children were present were more powerful predictors of…
-
Diabetic nephropathy and antioxidants.
Tavafi, Majid
2013-01-01
Oxidative stress has crucial role in pathogenesis of diabetic nephropathy (DN). Despite satisfactory results from antioxidant therapy in rodent, antioxidant therapy showed conflicting results in combat with DN in diabetic patients. Directory of Open Access Journals (DOAJ), Google Scholar,Pubmed (NLM), LISTA (EBSCO) and Web of Science have been searched. Treatment of DN in human are insufficient with rennin angiotensin system (RAS) blockers, so additional agent ought to combine with this management. Meanwhile based on DN pathogenesis and evidences in experimental and human researches, the antioxidants are the best candidate. New multi-property antioxidants may be improved human DN that show high power antioxidant capacity, long half-life time, high permeability to mitochondrion, improve body antioxidants enzymes activity and anti-inflammatory effects. Based on this review and our studies on diabetic rats, rosmarinic acid a multi-property antioxidant may be useful in DN patients, but of course, needs to be proven in clinical trials studies.
-
Solar multi-conjugate adaptive optics performance improvement
Zhang, Zhicheng; Zhang, Xiaofang; Song, Jie
2015-08-01
In order to overcome the effect of the atmospheric anisoplanatism, Multi-Conjugate Adaptive Optics (MCAO), which was developed based on turbulence correction by means of several deformable mirrors (DMs) conjugated to different altitude and by which the limit of a small corrected FOV that is achievable with AO is overcome and a wider FOV is able to be corrected, has been widely used to widen the field-of-view (FOV) of a solar telescope. With the assistance of the multi-threaded Adaptive Optics Simulator (MAOS), we can make a 3D reconstruction of the distorted wavefront. The correction is applied by one or more DMs. This technique benefits from information about atmospheric turbulence at different layers, which can be used to reconstruct the wavefront extremely well. In MAOS, the sensors are either simulated as idealized wavefront gradient sensors, tip-tilt sensors based on the best Zernike fit, or a WFS using physical optics and incorporating user specified pixel characteristics and a matched filter pixel processing algorithm. Only considering the atmospheric anisoplanatism, we focus on how the performance of a solar MCAO system is related to the numbers of DMs and their conjugate heights. We theoretically quantify the performance of the tomographic solar MCAO system. The results indicate that the tomographic AO system can improve the average Strehl ratio of a solar telescope by only employing one or two DMs conjugated to the optimum altitude. And the S.R. has a significant increase when more deformable mirrors are used. Furthermore, we discuss the effects of DM conjugate altitude on the correction achievable by the MCAO system, and present the optimum DM conjugate altitudes.
-
Site-Selective Conjugation of Native Proteins with DNA
DEFF Research Database (Denmark)
Trads, Julie Brender; Tørring, Thomas; Gothelf, Kurt Vesterager
2017-01-01
Conjugation of DNA to proteins is increasingly used in academia and industry to provide proteins with tags for identification or handles for hybridization to other DNA strands. Assay technologies such as immuno-PCR and proximity ligation and the imaging technology DNA-PAINT require DNA-protein....... The introduction of a bioorthogonal handle at a specific position of a protein by recombinant techniques provides an excellent approach to site-specific conjugation, but for many laboratories and for applications where several proteins are to be labeled, the expression of recombinant proteins may be cumbersome...... conjugates. In DNA nanotechnology, the DNA handle is exploited to precisely position proteins by self-assembly. For these applications, site-selective conjugation is almost always desired because fully functional proteins are required to maintain the specificity of antibodies and the activity of enzymes...
-
Conjugated Polymers for Flexible Energy Harvesting and Storage.
Zhang, Zhitao; Liao, Meng; Lou, Huiqing; Hu, Yajie; Sun, Xuemei; Peng, Huisheng
2018-03-01
Since the discovery of conjugated polymers in the 1970s, they have attracted considerable interest in light of their advantages of having a tunable bandgap, high electroactivity, high flexibility, and good processability compared to inorganic conducting materials. The above combined advantages make them promising for effective energy harvesting and storage, which have been widely studied in recent decades. Herein, the key advancements in the use of conjugated polymers for flexible energy harvesting and storage are reviewed. The synthesis, structure, and properties of conjugated polymers are first summarized. Then, their applications in flexible polymer solar cells, thermoelectric generators, supercapacitors, and lithium-ion batteries are described. The remaining challenges are then discussed to highlight the future direction in the development of conjugated polymers. © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
-
Lipke, Elisabeth; Michalik, Peter
2012-11-01
Sperm conjugation, where two or more sperm are physically united, is a rare but widespread pheno-menon across the animal kingdom. One group well known for its different types of sperm conjugation are spiders. Particularly, haplogyne spiders show a high diversity of sperm traits. Besides individual cleistospermia, primary (synspermia) and secondary (coenospermia, "spermatophore") sperm conjugation occurs. However, the evolution of sperm conjugates and sperm is not understood in this group. Here, we look at how sperm are transferred in Caponiidae (Haplogynae) in pursuit of additional information about the evolution of sperm transfer forms in spiders. Additionally, we investigated the male reproductive system and spermatozoa using light- and transmission electron-microscopy and provide a 3D reconstruction of individual as of well as conjugated spermatozoa. Mature spermatozoa are characterized by an extremely elongated, helical nucleus resulting in the longest spider sperm known to date. At the end of spermiogenesis, synspermia are formed by complete fusion of four spermatids. Thus, synspermia might have evolved early within ecribellate Haplogynae. The fused sperm cells are surrounded by a prominent vesicular area. The function of the vesicular area remains still unknown but might be correlated with the capacitation process inside the female. Further phylogenetic and functional implications of the spermatozoa and sperm conjugation are discussed. Copyright © 2012 Elsevier Ltd. All rights reserved.
-
Soluble Antioxidant Compounds Regenerate the Antioxidants Bound to Insoluble Parts of Foods
Celik, E.E.; Gökmen, V.; Fogliano, V.
2013-01-01
This study aimed to investigate the regeneration potential of antioxidant capacity of an insoluble food matrix. Investigations were performed in vitro with several food matrices rich in dietary fiber (DF) and bound antioxidants. After removal of the soluble fraction, the antioxidant capacity (AC) of
-
Design and biological activity of β-sheet breaker peptide conjugates
International Nuclear Information System (INIS)
Rocha, Sandra; Cardoso, Isabel; Boerner, Hans; Pereira, Maria Carmo; Saraiva, Maria Joao; Coelho, Manuel
2009-01-01
The sequence LPFFD (iAβ 5 ) prevents amyloid-β peptide (Aβ) fibrillogenesis and neurotoxicity, hallmarks of Alzheimer's disease (AD), as previously demonstrated. In this study iAβ 5 was covalently linked to poly(ethylene glycol) (PEG) and the activity of conjugates was assessed and compared to the activity of the peptide alone by in vitro studies. The conjugates were characterized by MALDI-TOF. Competition binding assays established that conjugates retained the ability to bind Aβ with similar strength as iAβ 5 . Transmission electron microscopy analysis showed that iAβ 5 conjugates inhibited amyloid fibril formation, which is in agreement with binding properties observed for the conjugates towards Aβ. The conjugates were also able to prevent amyloid-induced cell death, as evaluated by activation of caspase 3. These results demonstrated that the biological activity of iAβ 5 is not affected by the pegylation process.
-
Antiradical and antioxidant activities of new bio-antioxidants.
Kancheva, V D; Saso, L; Angelova, S E; Foti, M C; Slavova-Kasakova, A; Daquino, C; Enchev, V; Firuzi, O; Nechev, J
2012-02-01
Antioxidants could be promising agents for management of oxidative stress-related diseases. New biologically active compounds, belonging to a rare class of natural lignans with antiangiogenic, antitumoral and DNA intercalating properties, have been recently synthesized. These compounds are benzo[kl]xanthene lignans (1,2) and dihydrobenzofuran neolignans (3,4). The radical scavenging and chain-breaking antioxidant activities of compounds 1-4 were studied by applying different methods: radical scavenging activity by DPPH rapid test, chain-breaking antioxidant activity and quantum chemical calculations. All studied compounds were found to be active as DPPH scavengers but reaction time with DPPH and compounds' concentrations influenced deeply the evaluation. The highest values of radical scavenging activity (%RSAmax) and largest rate constants for reaction with DPPH were obtained for compounds 2 and 3. Comparison of %RSAmax with that of standard antioxidants DL-α-tocopherol (TOH), caffeic acid (CA) and butylated hydroxyl toluene (BHT) give the following new order of %RSA max: TOH (61.1%) > CA (58.6%) > 3 (36.3%) > 2 (28.1%) > 4 (6.7%) > 1 (3.6%) = BHT (3.6%). Chain-breaking antioxidant activities of individual compounds (0.1-1.0 mM) and of their equimolar binary mixtures (0.1 mM) with TOH were determined from the kinetic curves of lipid autoxidation at 80 °C. On the basis of a comparable kinetic analysis with standard antioxidants a new order of the antioxidant efficiency (i.e., protection factor, PF) of compounds 1-4 were obtained: 2 (7.2) ≥ TOH (7.0) ≥ CA (6.7) > 1 (3.1) > 3 (2.2) > ferulic acid FA (1.5) > 4 (0.6); and of the antioxidant reactivity (i.e. inhibition degree, ID): 2 (44.0) > TOH (18.7) > CA (9.3) > 1 (8.4) > 3 (2.8) > FA (1.0) > 4 (0.9). The important role of the catecholic structure in these compounds, which is responsible for the high chain-breaking antioxidant activity, is discussed and a reaction
-
Poulin-Laprade, Dominic; Carraro, Nicolas; Burrus, Vincent
2015-01-01
Nowadays, healthcare systems are challenged by a major worldwide drug resistance crisis caused by the massive and rapid dissemination of antibiotic resistance genes and associated emergence of multidrug resistant pathogenic bacteria, in both clinical and environmental settings. Conjugation is the main driving force of gene transfer among microorganisms. This mechanism of horizontal gene transfer mediates the translocation of large DNA fragments between two bacterial cells in direct contact. Integrative and conjugative elements (ICEs) of the SXT/R391 family (SRIs) and IncA/C conjugative plasmids (ACPs) are responsible for the dissemination of a broad spectrum of antibiotic resistance genes among diverse species of Enterobacteriaceae and Vibrionaceae. The biology, diversity, prevalence and distribution of these two families of conjugative elements have been the subject of extensive studies for the past 15 years. Recently, the transcriptional regulators that govern their dissemination through the expression of ICE- or plasmid-encoded transfer genes have been described. Unrelated repressors control the activation of conjugation by preventing the expression of two related master activator complexes in both types of elements, i.e., SetCD in SXT/R391 ICEs and AcaCD in IncA/C plasmids. Finally, in addition to activating ICE- or plasmid-borne genes, these master activators have been shown to specifically activate phylogenetically unrelated mobilizable genomic islands (MGIs) that also disseminate antibiotic resistance genes and other adaptive traits among a plethora of pathogens such as Vibrio cholerae and Salmonella enterica.
-
Directory of Open Access Journals (Sweden)
Dominic ePoulin-Laprade
2015-08-01
Full Text Available Nowadays, healthcare systems are challenged by a major worldwide drug resistance crisis caused by the massive and rapid dissemination of antibiotic resistance genes and associated emergence of multidrug resistant pathogenic bacteria, in both clinical and environmental settings. Conjugation is the main driving force of gene transfer among microorganisms. This mechanism of horizontal gene transfer mediates the translocation of large DNA fragments between two bacterial cells in direct contact. Integrative and conjugative elements (ICEs of the SXT/R391 family (SRIs and IncA/C conjugative plasmids (ACPs are responsible for the dissemination of a broad spectrum of antibiotic resistance genes among diverse species of Enterobacteriaceae and Vibrionaceae. The biology, diversity, prevalence and distribution of these two families of conjugative elements have been the subject of extensive studies for the past 15 years. Recently, the transcriptional regulators that govern their dissemination through the expression of ICE- or plasmid-encoded transfer genes have been described. Unrelated repressors control the activation of conjugation by preventing the expression of two related master activator complexes in both types of elements, i.e. SetCD in SXT/R391 ICEs and AcaCD in IncA/C plasmids. Finally, in addition to activating ICE- or plasmid-borne genes, these master activators have been shown to specifically activate phylogenetically unrelated mobilizable genomic islands (MGIs that also disseminate antibiotic resistance genes and other adaptive traits among a plethora of pathogens such as Vibrio cholerae and Salmonella enterica.
-
Antioxidant properties of catechins: Comparison with other antioxidants.
Grzesik, Michalina; Naparło, Katarzyna; Bartosz, Grzegorz; Sadowska-Bartosz, Izabela
2018-02-15
Antioxidant properties of five catechins and five other flavonoids were compared with several other natural and synthetic compounds and related to glutathione and ascorbate as key endogenous antioxidants in several in vitro tests and assays involving erythrocytes. Catechins showed the highest ABTS-scavenging capacity, the highest stoichiometry of Fe 3+ reduction in the FRAP assay and belonged to the most efficient compounds in protection against SIN-1 induced oxidation of dihydrorhodamine 123, AAPH-induced fluorescein bleaching and hypochlorite-induced fluorescein bleaching. Glutathione and ascorbate were less effective. (+)-catechin and (-)-epicatechin were the most effective compounds in protection against AAPH-induced erythrocyte hemolysis while (-)-epicatechin gallate, (-)-epigallocatechin gallate and (-)-epigallocatechin protected at lowest concentrations against hypochlorite-induced hemolysis. Catechins [(-)-epigallocatechin gallate and (-)-epicatechin gallate)] were most efficient in the inhibition of AAPH-induced oxidation of 2'7'-dichlorodihydroflurescein contained inside erythrocytes. Excellent antioxidant properties of catechins and other flavonoids make them ideal candidates for nanoformulations to be used in antioxidant therapy. Copyright © 2017 Elsevier Ltd. All rights reserved.
-
Martini, Daniela; Ciccoritti, Roberto; Nicoletti, Isabella; Nocente, Francesca; Corradini, Danilo; D'Egidio, Maria Grazia; Taddei, Federica
2018-02-01
The aim of this work was to compare the traditional with a non-conventional (i.e. kernel micronisation) durum wheat milling process by monitoring the content of bound, conjugated and free phenolic acids (PAs) and the level of the total antioxidant capacity (TAC) occurring in the durum wheat pasta production chain, from seed to cooked pasta. The traditional transformation processes negatively influenced TAC and PA content (40% and 89% decrease from seed to cooked pasta, respectively), mainly during the milling process (25% and 84% decrease of TAC and PA, respectively), which has been related to the removal of external layers of kernels. Conversely, the micronisation applied on durum wheat kernels allowed to obtain whole-wheat pasta that preserved the seed endowment of antioxidant compounds even in cooked pasta. These results indicate the micronisation as a valuable approach to produce pasta with improved nutritional value and potential health-promoting effects compared to the traditional pasta.
-
Synthesis and antimicrobial activity of gold nanoparticle conjugates with cefotaxime
Titanova, Elena O.; Burygin, Gennady L.
2016-04-01
Gold nanoparticles (GNPs) have attracted significant interest as a novel platform for various applications to nanobiotechnology and biomedicine. The conjugates of GNPs with antibiotics and antibodies were also used for selective photothermal killing of protozoa and bacteria. Also the conjugates of some antibiotics with GNPs decreased the number of bacterial growing cells. In this work was made the procedure optimization for conjugation of cefotaxime (a third-generation cephalosporin antibiotic) with GNPs (15 nm) and we examined the antimicrobial properties of this conjugate to bacteria culture of E. coli K-12. Addition of cefotaxime solution to colloidal gold does not change their color and extinction spectrum. For physiologically active concentration of cefotaxime (3 μg/mL), it was shown that the optimum pH for the conjugation was more than 9.5. A partial aggregation of the GNPs in saline medium was observed at pH 6.5-7.5. The optimum concentration of K2CO3 for conjugation cefotaxime with GNPs-15 was 5 mM. The optimum concentration of cefotaxime was at 0.36 μg/mL. We found the inhibition of the growth of E. coli K12 upon application cefotaxime-GNP conjugates.
-
Acute Alcohol Consumption Elevates Serum Bilirubin, an Endogenous Antioxidant
O’Malley, Stephanie S.; Gueorguieva, Ralitza; Wu, Ran; Jatlow, Peter I.
2015-01-01
Background Moderate alcohol consumption has been associated with both negative and favorable effects on health. The mechanisms responsible for reported favorable effects remain unclear. Higher (not necessarily elevated) concentrations of serum bilirubin, an antioxidant, have also been associated with reduced risk of cardiovascular disease and all-cause mortality. This study tests the hypothesis that single dose alcohol consumption elevates bilirubin providing a potential link between these observations. Methods 18 healthy individuals (8 cigarette smokers) were administered alcohol, calibrated to achieve blood concentrations of 20, 80 and 120 mg/dL, in random order in 3 laboratory sessions separated by a week. Each session was preceded by and followed by 5–7 days of alcohol abstinence. Serum bilirubin was measured at 7:45 am prior to drinking, at 2 pm, and at 7:45 the next morning. Mixed effects regression models compared baseline and 24 hr. post-drinking bilirubin concentrations. Results Total serum bilirubin (sum of indirect and direct) concentration increased significantly after drinking from baseline to 24 hours in non-smokers (from Mean=0.38, SD=0.24 to Mean=0.51 SD=0.30, F(1, 32.2) =24.24, pbilirubin concentration and the ratio of indirect (unconjugated) to direct (conjugated) bilirubin also increased significantly. Conclusions Alcohol consumption leads to increases in serum bilirubin in nonsmokers. Considering the antioxidant properties of bilirubin, our findings suggest one possible mechanism for the reported association between alcohol consumption and reduced risk of some disorders that could be tested in future longitudinal studies. PMID:25707709
-
Conjugated Polymer Solar Cells
National Research Council Canada - National Science Library
Paraschuk, Dmitry Y
2006-01-01
This report results from a contract tasking Moscow State University as follows: Conjugated polymers are promising materials for many photonics applications, in particular, for photovoltaic and solar cell devices...
-
Soluble polymer conjugates for drug delivery.
Minko, Tamara
2005-01-01
The use of water-soluble polymeric conjugates as drug carriers offers several possible advantages. These advantages include: (1) improved drug pharmacokinetics; (2) decreased toxicity to healthy organs; (3) possible facilitation of accumulation and preferential uptake by targeted cells; (4) programmed profile of drug release. In this review, we will consider the main types of useful polymeric conjugates and their role and effectiveness as carriers in drug delivery systems.: © 2005 Elsevier Ltd . All rights reserved.
-
Diffeomorphisms Holder conjugate to Anosov diffeomorphisms
Gogolev, Andrey
2008-01-01
We show by means of a counterexample that a $C^{1+Lip}$ diffeomorphism Holder conjugate to an Anosov diffeomorphism is not necessarily Anosov. The counterexample can bear higher smoothness up to $C^3$. Also we include a result from the 2006 Ph.D. thesis of T. Fisher: a $C^{1+Lip}$ diffeomorphism Holder conjugate to an Anosov diffeomorphism is Anosov itself provided that Holder exponents of the conjugacy and its inverse are sufficiently large.
-
Conjugate Problems in Convective Heat Transfer: Review
Directory of Open Access Journals (Sweden)
Abram Dorfman
2009-01-01
Full Text Available A review of conjugate convective heat transfer problems solved during the early and current time of development of this modern approach is presented. The discussion is based on analytical solutions of selected typical relatively simple conjugate problems including steady-state and transient processes, thermal material treatment, and heat and mass transfer in drying. This brief survey is accompanied by the list of almost two hundred publications considering application of different more and less complex analytical and numerical conjugate models for simulating technology processes and industrial devices from aerospace systems to food production. The references are combined in the groups of works studying similar problems so that each of the groups corresponds to one of selected analytical solutions considered in detail. Such structure of review gives the reader the understanding of early and current situation in conjugate convective heat transfer modeling and makes possible to use the information presented as an introduction to this area on the one hand, and to find more complicated publications of interest on the other hand.
-
Braakhuis, Andrea J; Hopkins, Will G; Lowe, Timothy E
2013-09-01
The beneficial effects of exercise and a healthy diet are well documented in the general population but poorly understood in elite athletes. Previous research in subelite athletes suggests that regular training and an antioxidant-rich diet enhance antioxidant defenses but not performance. To investigate whether habitual diet and/or exercise (training status or performance) affect antioxidant status in elite athletes. Antioxidant blood biomarkers were assessed before and after a 30-min ergometer time trial in 28 male and 34 female rowers. The antioxidant blood biomarkers included ascorbic acid, uric acid, total antioxidant capacity (TAC), erythrocyte- superoxide dismutase, glutathione peroxidase (GPx), and catalase. Rowers completed a 7-d food diary and an antioxidant-intake questionnaire. Effects of diet, training, and performance on resting biomarkers were assessed with Pearson correlations, and their effect on exercise-induced changes in blood biomarkers was assessed by a method of standardization. With the exception of GPx, there were small to moderate increases with exercise for all markers. Blood resting TAC had a small correlation with total antioxidant intake (correlation .29; 90% confidence limits, ±.27), and the exercise-induced change in TAC had a trivial to small association with dietary antioxidant intake from vitamin C (standardized effect .19; ±.22), vegetables (.20; ±.23), and vitamin A (.25; ±.27). Most other dietary intakes had trivial associations with antioxidant biomarkers. Years of training had a small inverse correlation with TAC (-.32; ±.19) and a small association with the exercise-induced change in TAC (.27; ±.24). Training status correlates more strongly with antioxidant status than diet does.
-
Improvement of Emulsifying Properties of Wheat Gluten Hydrolysate λ-Carrageenan Conjugates
Directory of Open Access Journals (Sweden)
Jin-Shui Wang
2006-01-01
Full Text Available Gluten hydrolysate was prepared through limited enzymatic hydrolysis of wheat gluten resulting from the byproducts of wheat starch. The enzyme applied in the present study was Protamex. Response surface methodology was used to investigate the effects of pH, gluten hydrolysate (GHPλ-carrageenan (C ratio and reaction time on emulsifying properties of the GHP-C conjugate. The regression model for emulsion activity index (EAI was significant at p=0.001, while reaction time had a significant effect on EAI of the conjugate with regression coefficient of 4.25. The interactions of pH and GHP/ C ratio, and GHP/C ratio and reaction time significantly affected the EAI of the conjugate. Both the emulsifying property and nitrogen solubility index (NSI of GHP-C conjugate prepared under the optimal conditions increased more remarkably, compared to the control. The denaturation temperature of GHP-C conjugate obviously increased compared to wheat gluten. The addition of GHP-C conjugate had different effects on dough characteristics. Moreover, this conjugate can delay the increase in the bread crumb firmness during storage. It demonstrated that this conjugate couldimprove the dough characteristics and had anti-staling properties of bread.
-
International Nuclear Information System (INIS)
Matern, S.; Gerok, W.
1977-01-01
Specific radioimmunoassays for separate determinations of serum unconjugated cholic, conjugated cholic and conjugated deoxycholic acids have been developed. Prior to the radioimmunoassay, extraction of serum bile acids was performed with Amberlite XAD-2. Unconjugated cholic acid was separated from glyco- and taurocholic acids by thin-layer chromatography. At 50% displacement of bound labeled glyco[ 3 H]cholic acid using antiserum obtained after immunization with cholic acid-bovine serum albumin-conjugate the cross-reactivity of taurocholic acid was 100%, cholic acid 80%, glycochenodeoxycholic acid 10%, chenodeoxycholic acid 7%, conjugated deoxycholic acid 3%, and conjugated lithocholic acid 3 H]cholic acid was linear on a logit-log plot from 5 to 80 pmol of unlabeled glycocholic acid. Fasting serum conjugated cholic acid in healthy subjects was 0.68 +- 0.34 μmol/l. Unconjugated cholic acid was determined by a solid phase radioimmunoassay using the cholic acid antibody chemically bound to Sepharose. The displacement curve of [ 3 H]cholic acid in the solid phase radioimmunoassay was linear on a logit-log plot from 5 to 200 pmol of unlabeled cholic acid. The coefficient of variation between samples was 5%. Fasting serum conjugated deoxycholic acid concentrations in 10 healthy subjects ranged from 0.18 to 0.92 μmol/l determined by a radioimmunoassay using antiserum obtained after immunization with deoxycholic acid-bovine serum albumin-conjugate. The clinical application of these bile acid radioimmunoassays is shown by an 'oral cholate tolerance test' as a sensitive indicator of liver function and by an 'oral cholyglycine tolerance test' as a useful test for bile acid absorption. (orig.) [de
-
Vogel, Curtis R; Yang, Qiang
2006-08-21
We present two different implementations of the Fourier domain preconditioned conjugate gradient algorithm (FD-PCG) to efficiently solve the large structured linear systems that arise in optimal volume turbulence estimation, or tomography, for multi-conjugate adaptive optics (MCAO). We describe how to deal with several critical technical issues, including the cone coordinate transformation problem and sensor subaperture grid spacing. We also extend the FD-PCG approach to handle the deformable mirror fitting problem for MCAO.
-
Preparation and biodistribution study of 99Tcm labelled dextran conjugates
International Nuclear Information System (INIS)
Yang Chunhui; Li Hongyu; Liang Jixin; Lu Jia; Luo Hongyi; Zheng Deqiang; Sun Guiquan
2012-01-01
99 Tc m Mannosylated dextran conjugates were prepared through [ 99 Tc m (CO) 3 ] + precursor synthesized by carbonyl Isolink kit. The labelled conjugates were injected sub-dermally into the rear foots of the mice, and the patent blue solution was injected at the same site 10 min before sacrifice. The mice were killed at 1 h and 4 h postinjection, and the samples of different tissues including SLN, 2LN, injection site, liver, spleen, blood were dissected and counted. The uptake in terms was calculated. The results of biodistribution demonstrated that the SLN uptakes of radiopharmaceutical (without mannose in the molecules) were rather low and in vivo excretion of these conjugates were comparatively faster, and the uptake of injection site was also low; on the other hand, the SLN uptakes of radio pharmaceutical (with mannose in their molecules) were much higher than those of their corresponding dextran conjugates without mannose, but the retention in the injection site of these conjugates increased too. The results indicated that the affinity of mannosyl-dextran conjugates to the receptors on the surface of macrophages in the lymph node. In addition, the different relative molecular mass of dextran conjugates also cause different biodistribution results, the major one had higher SLN uptake, the difference was significant (P 99 Tc m (CO) 3 ] + labelled mannosylated dextran conjugates showed promising properties as SLN imaging agent and worth further investigation. (authors)
-
Developing SyrinOX total antioxidant capacity assay for measuring antioxidants in humans.
Prasetyo, Endry N; Knes, Otto; Nyanhongo, Gibson S; Guebitz, Georg M
2013-02-01
Accurate monitoring of the antioxidant status or of oxidative stress in patients is still a big challenge in clinical laboratories. This study investigates the possibility of applying a newly developed total antioxidant capacity assay method based on laccase or peroxidase oxidized syringaldazine [Tetramethoxy azobismethylene quinone (TMAMQ)] which is referred to here as SyrinOX, as a diagnostic tool for monitoring both oxidative stress and antioxidant status in patients. Attempts to adapt the Randox total antioxidant procedure [simultaneous incubation of the radical generating system (metmyoglobin and H(2) O(2) ) and antioxidant sample] for SyrinOX were abandoned after it was discovered that the H(2) O(2) reacted with enzymatically generated TMAMQ and ABTS radicals at a rate of 6.4 × 10(-2) /μM/s and 5.7 × 10(-3) /μM/s respectively. Thus this study for the first time demonstrates the negative effects of H(2) O(2) in the Randox system. This leads to erroneous results because the total antioxidant values obtained are the sum of radicals reduced by antioxidants plus those reacting with the radical generating system. Therefore they should be avoided not only for this particular method but also when using other similar methods. Consequently, SyrinOX is best applied using a three-step approach involving, production of TMAMQ, recovery and purification (free from enzyme and other impurities) and then using TMAMQ for measuring the total antioxidant capacity of samples. Using this approach, the reaction conditions for application of SyrinOX when measuring the total antioxidant capacity of plasma sample were determined to be 50% (v/v) ethanol/50 mM sodium succinate buffer pH 5.5, between 20 and 25 °C for at least 1 h. © 2012 The Authors. International Journal of Experimental Pathology © 2012 International Journal of Experimental Pathology.
-
International Nuclear Information System (INIS)
Zhang, K.; Zuo, Y.
2006-01-01
Phenolic compounds are the most abundant natural antioxidants in our diet. Epidemiological studies have shown the possible prevention effects of consumption of fruits and vegetables rich in phenolic compounds on degenerative diseases, such as cardiovascular diseases and cancers. However, there is a serious lack of fundamental knowledge on the uptake and metabolism of phenolic compounds in humans. It is clear that phenolic molecules, only absorbed by humans, can exert biological effects. This review presents a current knowledge on the analytical methods, antioxidant capacity measurements, as well as research strategies related to natural phenolic antioxidants on human health. Both GC-MS and LC-MS have proved to be very useful analytical techniques that can be employed to identify and quantitate targeted phenolic antioxidants and their metabolites in biofluids. Free radical quenching tests provide a direct measurement of antioxidant capacity but lack specificity and may oversimplify the in vivo human physiological environment. Research strategies are diverse and mainly focused on positive health effect of antioxidants. In the future studies, multiple potential bioactivities, both positive and negative, should be considered. (author)
-
Natural antioxidants in chemoprevention
Energy Technology Data Exchange (ETDEWEB)
Dragsted, L.O. [Danish Veterinary and Food Administration, Soeberg (Denmark). Inst. of Toxicology
1998-12-31
It is well documented that diets rich in fruits and vegetables can reduce the risk of most common cancers, and that some food items from this class may be protective against heart disease. Several explanations have been offered, one of which relates to the natural presence of potent antioxidants in plant products. Destructive oxidation of lipids, proteins, DNA, and other important biomolecules, often involving radical chain reactions, affect vital cellular structures and their normal functions. Such processes are involved in the development of cancer as well as heart disease, and it seems logical to assume that antioxidants might be preventive. Large human trials with natural antioxidants have not provided a uniform support, however, for the hypothesis that antioxidation per se may prevent cancer or coronary heart disease (CHD). One reason is that other effects, unrelated to antioxidation, may compromise their preventive effects. Another reason may be that many potent antioxidants can also act as pro-oxidants under certain conditions. The interpretation of animal trials is likewise often compromised by the fact that most antioxidants have other physiological effects which might very well explain their protective action or lead to toxic side-effects. (orig.)
-
Cross-conjugation and quantum interference: a general correlation?
DEFF Research Database (Denmark)
Valkenier, Hennie; Guedon, Constant M.; Markussen, Troels
2014-01-01
We discuss the relationship between the pi-conjugation pattern, molecular length, and charge transport properties of molecular wires, both from an experimental and a theoretical viewpoint. Specifically, we focus on the role of quantum interference in the conductance properties of cross-conjugated...
-
Energy Technology Data Exchange (ETDEWEB)
Baran, Erkan T., E-mail: erkantur@metu.edu.tr; Tuzlakoglu, Kadriye, E-mail: kadriye@dep.uminho.pt; Mano, Joao F., E-mail: jmano@dep.uminho.pt; Reis, Rui L., E-mail: rgreis@dep.uminho.pt
2012-08-01
The objective of this study was to investigate the influence of silk fibroin and oxidized starch conjugation on the enzymatic degradation behavior and the cytocompatability of chitosan based biomaterials. The tensile stress of conjugate membranes, which was at 50 Megapascal (MPa) for the lowest fibroin and starch composition (10 weight percent (wt.%)), was decreased significantly with the increased content of fibroin and starch. The weight loss of conjugates in {alpha}-amylase was more notable when the starch concentration was the highest at 30 wt.%. The conjugates were resistant to the degradation by protease and lysozyme except for the conjugates with the lowest starch concentration. After 10 days of cell culture, the proliferation of osteoblast-like cells (SaOS-2) was stimulated significantly by higher fibroin compositions and the DNA synthesis on the conjugate with the highest fibroin (30 wt.%) was about two times more compared to the native chitosan. The light microscopy and the image analysis results showed that the cell area and the lengths were decreased significantly with higher fibroin/chitosan ratio. The study proved that the conjugation of fibroin and starch with the chitosan based biomaterials by the use of non-toxic reductive alkylation crosslinking significantly improved the cytocompatibility and modulated the biodegradation, respectively. - Highlights: Black-Right-Pointing-Pointer Silk fibroin, starch and chitosan conjugates were prepared by reductive alkylation. Black-Right-Pointing-Pointer The enzymatic biodegradation and the cytocompatibility of conjugates were tested. Black-Right-Pointing-Pointer The conjugate with 30% starch composition was degraded by {alpha}-amylase significantly. Black-Right-Pointing-Pointer Higher starch composition in conjugates prevented protease and lysozyme degradation. Black-Right-Pointing-Pointer Fibroin incorporation effectively increased the cell proliferation of conjugates.
-
Recent Advances in Conjugated Polymers for Light Emitting Devices
AlSalhi, Mohamad Saleh; Alam, Javed; Dass, Lawrence Arockiasamy; Raja, Mohan
2011-01-01
A recent advance in the field of light emitting polymers has been the discovery of electroluminescent conjugated polymers, that is, kind of fluorescent polymers that emit light when excited by the flow of an electric current. These new generation fluorescent materials may now challenge the domination by inorganic semiconductor materials of the commercial market in light-emitting devices such as light-emitting diodes (LED) and polymer laser devices. This review provides information on unique properties of conjugated polymers and how they have been optimized to generate these properties. The review is organized in three sections focusing on the major advances in light emitting materials, recent literature survey and understanding the desirable properties as well as modern solid state lighting and displays. Recently, developed conjugated polymers are also functioning as roll-up displays for computers and mobile phones, flexible solar panels for power portable equipment as well as organic light emitting diodes in displays, in which television screens, luminous traffic, information signs, and light-emitting wallpaper in homes are also expected to broaden the use of conjugated polymers as light emitting polymers. The purpose of this review paper is to examine conjugated polymers in light emitting diodes (LEDs) in addition to organic solid state laser. Furthermore, since conjugated polymers have been approved as light-emitting organic materials similar to inorganic semiconductors, it is clear to motivate these organic light-emitting devices (OLEDs) and organic lasers for modern lighting in terms of energy saving ability. In addition, future aspects of conjugated polymers in LEDs were also highlighted in this review. PMID:21673938
-
The proper time for antioxidant consumption.
Beaulieu, Michaël; Schaefer, H Martin
2014-04-10
Consuming food rich in antioxidants may help organisms to increase their antioxidant defences and avoid oxidative damage. Under the hypothesis that organisms actively consume food for its antioxidant properties, they would need to do so in view of other physiological requirements, such as energy requirements. Here, we observed that Gouldian finches (Erythrura gouldiae) consumed most seeds rich in antioxidants in the middle of the day, while their consumption of staple seeds more profitable in energy intake (and poor in antioxidants) was maximal in the morning and the evening. This consumption of seeds rich in antioxidants in the middle of the day may be explicable (1) because birds took advantage of a time window associated with relaxed energy requirements to ingest antioxidant resources, or (2) because birds consumed antioxidant resources as a response to the highest antioxidant requirements in the middle of the day. If the latter hypothesis holds true, having the possibility to ingest antioxidants should be most beneficial in terms of oxidative balance in the middle of the day. Even though feeding on seeds rich in antioxidants improved Gouldian finches' overall antioxidant capacity, we did not detect any diurnal effect of antioxidant intake on plasma oxidative markers (as measured by the d-ROM and the OXY-adsorbent tests). This indicates that the diurnal pattern of antioxidant intake that we observed was most likely constrained by the high consumption of staple food to replenish or build up body reserves in the morning and in the evening, and not primarily determined by elevated antioxidant requirements in the middle of the day. Consequently, animals appear to have the possibility to increase antioxidant defences by selecting food rich in antioxidants, only when energetic constraints are relaxed. Copyright © 2014 Elsevier Inc. All rights reserved.
-
Energy Technology Data Exchange (ETDEWEB)
Phung, Thu-Ha; Jung, Sunyo, E-mail: sjung@knu.ac.kr
2015-04-03
This study focuses on differential molecular mechanisms of antioxidant and detoxification systems in rice plants under two different types of photodynamic stress imposed by porphyrin deregulators, 5-aminolevulinic acid (ALA) and oxyfluorfen (OF). The ALA-treated plants with white necrosis exhibited a greater decrease in photochemical quantum efficiency, F{sub v}/F{sub m}, as well as a greater increase in activity of superoxide dismutase, compared to the OF-treated plants. By contrast, the brown necrosis in OF-treated plants resulted in not only more widely dispersed H{sub 2}O{sub 2} production and greater increases in H{sub 2}O{sub 2}-decomposing enzymes, catalase and peroxidase, but also lower ascorbate redox state. In addition, ALA- and OF-treated plants markedly up-regulated transcript levels of genes involved in detoxification processes including transport and movement, cellular homeostasis, and xenobiotic conjugation, with prominent up-regulation of serine/threonine kinase and chaperone only in ALA-treated plants. Our results demonstrate that different photodynamic stress imposed by ALA and OF developed differential actions of antioxidant enzymes and detoxification. Particularly, detoxification system may play potential roles in plant protection against photodynamic stress imposed by porphyrin deregulators, thereby contributing to alleviation of photodynamic damage. - Highlights: • We employ two different types of photodynamic stress, white and brown necrosis. • We examine molecular mechanisms of antioxidative and detoxification systems. • ALA and OF develop differential actions of antioxidant and detoxification systems. • Coordinated mechanism of antioxidants and detoxification works against toxic ROS. • Detoxification system plays critical roles in protection against photodynamic stress.
-
International Nuclear Information System (INIS)
Phung, Thu-Ha; Jung, Sunyo
2015-01-01
This study focuses on differential molecular mechanisms of antioxidant and detoxification systems in rice plants under two different types of photodynamic stress imposed by porphyrin deregulators, 5-aminolevulinic acid (ALA) and oxyfluorfen (OF). The ALA-treated plants with white necrosis exhibited a greater decrease in photochemical quantum efficiency, F v /F m , as well as a greater increase in activity of superoxide dismutase, compared to the OF-treated plants. By contrast, the brown necrosis in OF-treated plants resulted in not only more widely dispersed H 2 O 2 production and greater increases in H 2 O 2 -decomposing enzymes, catalase and peroxidase, but also lower ascorbate redox state. In addition, ALA- and OF-treated plants markedly up-regulated transcript levels of genes involved in detoxification processes including transport and movement, cellular homeostasis, and xenobiotic conjugation, with prominent up-regulation of serine/threonine kinase and chaperone only in ALA-treated plants. Our results demonstrate that different photodynamic stress imposed by ALA and OF developed differential actions of antioxidant enzymes and detoxification. Particularly, detoxification system may play potential roles in plant protection against photodynamic stress imposed by porphyrin deregulators, thereby contributing to alleviation of photodynamic damage. - Highlights: • We employ two different types of photodynamic stress, white and brown necrosis. • We examine molecular mechanisms of antioxidative and detoxification systems. • ALA and OF develop differential actions of antioxidant and detoxification systems. • Coordinated mechanism of antioxidants and detoxification works against toxic ROS. • Detoxification system plays critical roles in protection against photodynamic stress
-
Some aspects of geomagnetically conjugate phenomena
Energy Technology Data Exchange (ETDEWEB)
Rycroft, M.J.
1987-12-01
Both charged particles and waves convey information about the thermosphere, ionosphere and magnetosphere from the Northern to the Southern Hemisphere and vice versa, along geomagnetic flux tubes.The interhemispheric travel time of electrons or ions, being dependent upon L-value , pitch angle and energy (which may lie between less than or equal to 1 eV and greater than or equal to 1 MeV) may be many hours, ranging down to less than or equal to 1 s. However, the one-hop propagation time for magnetohydrodynamic or whistler mode waves generally lies between 10/sup 2/s and 1 s. Such times, therefore, give the time scales of transient phenomena that are geomagnetically conjugate and of changes in steady-state plasma processes occurring in geomagnetically conjugate regions. Contrasting examples are presented of conjugate physical phenomena, obtained using satellite, rocket, aircraft and ground-based observations; the latter capitalise upon the rather rare disposition of land - rather than ocean - at each end of a geophysically interesting flux tube. Particular attention is paid to the interactions between whistler mode waves and energetic electrons. Geomagnetic, radio, optical and plasma observations, taken together with model computations, provide a wealth of knowledge on conjugate phenomena and their dependence on conditions in the solar wind, substorms, L-value, etc... Finally, some suggestions are made for future lines of research.
-
Comparative cytotoxicity of gold-doxorubicin and InP-doxorubicin conjugates.
Zhang, Xuan; Chibli, Hicham; Kong, Dekun; Nadeau, Jay
2012-07-11
Direct comparisons of different types of nanoparticles for drug delivery have seldom been performed. In this study we compare the physical properties and cellular activity of doxorubicin (Dox) conjugates to gold nanoparticles (Au) and InP quantum dots of comparable diameter. Although the Au particles alone are non-toxic and InP is moderately toxic, Au-Dox is more effective than InP-Dox against the Dox-resistant B16 melanoma cell line. Light exposure does not augment the efficacy of InP-Dox, suggesting that conjugates are breaking down. Electron and confocal microscopy and atomic absorption spectroscopy reveal that over 60% of the Au-Dox conjugates reach the cell nucleus. In contrast, InP-Dox enters cell nuclei to a very limited extent, although liberated Dox from the conjugates does eventually reach the nucleus. These observations are attributed to faster Dox release from Au conjugates under endosomal conditions, greater aggregation of InP-Dox with cytoplasmic proteins, and adherence of InP to membranes. These findings have important implications for design of active drug-nanoparticle conjugates.
-
Anticancer activity of drug conjugates in head and neck cancer cells.
Majumdar, Debatosh; Rahman, Mohammad Aminur; Chen, Zhuo Georgia; Shin, Dong M
2016-06-01
Sexually transmitted oral cancer/head and neck cancer is increasing rapidly. Human papilloma virus (HPV) is playing a role in the pathogenesis of a subset of squamous cell carcinoma of head and neck (SCCHN). Paclitaxel is a widely used anticancer drug for breast, ovarian, testicular, cervical, non-small cell lung, head and neck cancer. However, it is water insoluble and orally inactive. We report the synthesis of water soluble nanosize conjugates of paclitaxel, branched PEG, and EGFR-targeting peptide by employing native chemical ligation. We performed a native chemical ligation between the N-hydroxy succinimide (NHS) ester of paclitaxel succinate and cysteine at pH 6.5 to give the cysteine-conjugated paclitaxel derivative. The thiol functionality of cysteine was activated and subsequently conjugated to multiarm thiol-PEG to obtain the paclitaxel branched PEG conjugate. Finally, we conjugated an EGFR-targeting peptide to obtain conjugates of paclitaxel, branched PEG, and EGFR-targeting peptide. These conjugates show anticancer activity against squamous cell carcinoma of head and neck cells (SCCHN, Tu212).
-
Small angle scattering from protein/sugar conjugates
Jackson, Andrew; White, John
2006-11-01
The Maillard reaction between free amine groups on proteins and sugars is well known. We have examined the effect of the reaction of the casein group of milk proteins with sugars on their nanoscale structure and aggregation. The small angle neutron scattering from beta casein and sodium caseinate and their sugar conjugates have been studied as a function of solution concentration. At high conjugate concentration (greater than ca. 5 mg/ml) the addition of sugar reduces supra-micellar aggregation of the protein whilst at lower concentration, where the protein is expected to be deaggregated already, little effect is seen. Guinier analysis of the scattering data show a radius of gyration of around 75 A˚ for beta casein in solution and around 80 A˚ for the sucrose conjugate.
-
Poljsak, Borut; Dahmane, Raja; Godic, Aleksandar
2013-04-01
It is estimated that total sun exposure occurs non-intentionally in three quarters of our lifetimes. Our skin is exposed to majority of UV radiation during outdoor activities, e.g. walking, practicing sports, running, hiking, etc. and not when we are intentionally exposed to the sun on the beach. We rarely use sunscreens during those activities, or at least not as much and as regular as we should and are commonly prone to acute and chronic sun damage of the skin. The only protection of our skin is endogenous (synthesis of melanin and enzymatic antioxidants) and exogenous (antioxidants, which we consume from the food, like vitamins A, C, E, etc.). UV-induced photoaging of the skin becomes clinically evident with age, when endogenous antioxidative mechanisms and repair processes are not effective any more and actinic damage to the skin prevails. At this point it would be reasonable to ingest additional antioxidants and/or to apply them on the skin in topical preparations. We review endogenous and exogenous skin protection with antioxidants.
-
Quantum dot-polymer conjugates for stable luminescent displays.
Ghimire, Sushant; Sivadas, Anjaly; Yuyama, Ken-Ichi; Takano, Yuta; Francis, Raju; Biju, Vasudevanpillai
2018-05-23
The broad absorption of light in the UV-Vis-NIR region and the size-based tunable photoluminescence color of semiconductor quantum dots make these tiny crystals one of the most attractive antennae in solar cells and phosphors in electrooptical devices. One of the primary requirements for such real-world applications of quantum dots is their stable and uniform distribution in optically transparent matrices. In this work, we prepare transparent thin films of polymer-quantum dot conjugates, where CdSe/ZnS quantum dots are uniformly distributed at high densities in a chitosan-polystyrene copolymer (CS-g-PS) matrix. Here, quantum dots in an aqueous solution are conjugated to the copolymer by a phase transfer reaction. With the stable conjugation of quantum dots to the copolymer, we prevent undesired phase separation between the two and aggregation of quantum dots. Furthermore, the conjugate allows us to prepare transparent thin films in which quantum dots are uniformly distributed at high densities. The CS-g-PS copolymer helps us in not only preserving the photoluminescence properties of quantum dots in the film but also rendering excellent photostability to quantum dots at the ensemble and single particle levels, making the conjugate a promising material for photoluminescence-based devices.
-
Free and conjugated dopamine in human ventricular fluid
International Nuclear Information System (INIS)
Sharpless, N.S.; Thal, L.J.; Wolfson, L.I.; Tabaddor, K.; Tyce, G.M.; Waltz, J.M.
1981-01-01
Free dopamine and an acid hydrolyzable conjugate of dopamine were measured in human ventricular fluid specimens with a radioenzymatic assay and by high performance liquid chromatography (HPLC) with electrochemical detection. Only trace amounts of free norepinephrine and dopamine were detected in ventricular fluid from patients with movement disorders. When the ventricular fluid was hydrolyzed by heating in HClO 4 or by lyophilization in dilute HClO 4 , however, a substantial amount of free dopamine was released. Values for free plus conjugated dopamine in ventricular fluid from patients who had never taken L-DOPA ranged from 139 to 340 pg/ml when determined by HPLC and from 223 to 428 pg/ml when measured radioenzymatically. The correlation coefficient for values obtained by the two methods in the same sample of CSF was 0.94 (P<0.001). Patients who had been treated with L-DOPA had higher levels of conjugated dopamine in their ventricular CSF which correlated inversely with the time between the last dose of L-DOPA and withdrawal of the ventricular fluid. Additionally, one patient with acute cerebral trauma had elevated levels of free norepinephrine and both free and conjugated dopamine in his ventricular fluid. Conjugation may be an important inactivation pathway for released dopamine in man. (Auth.)
-
Antibody-radioisotope conjugates for tumor localization and treatment
International Nuclear Information System (INIS)
Larson, S.M.; Carrasquillo, J.A.
1985-01-01
In principle, anti-tumor antibodies can be used to carry radioactivity to tumors for in-vivo diagnosis and treatment of cancer. First, for diagnostic purposes, an antibody that targets a specific antigen (for example, the p97 antigen of human melanoma tumor), is labeled with a tracer amount of radioactivity. When this antibody-radioisotope conjugate is injected into the blood stream, the antibody carries the radioactivity throughout the body and in time, percolates through all the tissues of the body. Because the tumor has specific antigens to which the antibody can bind, the antibody conjugate progressively accumulates in the tumor. Using conventional nuclear medicine imaging equipment, the body of the patient is scanned for radioactivity content, and a map of the distribution of the radioactivity is displayed on photographic film. The tumor shows up as a dense area of radio-activity. These same antibody-radioisotope conjugates may be used for therapy of tumors, except that in this case large amounts of radioactivity are loaded on the antibody. After localization of the conjugate there is sufficient radiation deposited in the tumor of radiotherapy. The success of this approach in the clinic is determined in large measure by the concentration gradient that can be achieved between tissue antibody conjugate in tumor versus normal tissue
-
Conjugal conflict and violence: a review and theoretical paradigm.
Smilkstein, G; Aspy, C B; Quiggins, P A
1994-02-01
Conjugal violence has been described as having multiple etiologies. The variables are so numerous that intervention and research protocols are difficult to effect. This paper proposes a paradigm that establishes conjugal conflict and violence as separate entities. According to the paradigm, conjugal conflict is viewed as "an inevitable part of human association," whereas conjugal violence is determined to be a learned behavioral tactic that is employed as a coping strategy when an individual's conflict threshold potential is exceeded. Evidence will be offered that violence is learned from family of origin and from observing what is common or accepted practice in the community. Use of this paradigm would give primacy to community education programs that advance the concept of conflict resolution through rational discourse.
-
Hsiao, Shih-Chia; Francis, Matthew B.; Bertozzi, Carolyn; Mathies, Richard; Chandra, Ravi; Douglas, Erik; Twite, Amy; Toriello, Nicholas; Onoe, Hiroaki
2016-05-03
The present invention provides conjugates of DNA and cells by linking the DNA to a native functional group on the cell surface. The cells can be without cell walls or can have cell walls. The modified cells can be linked to a substrate surface and used in assay or bioreactors.
-
Hsiao, Shih-Chia; Francis, Matthew B.; Bertozzi, Carolyn; Mathies, Richard; Chandra, Ravi; Douglas, Erik; Twite, Amy; Toriello, Nicholas; Onoe, Hiroaki
2018-05-15
The present invention provides conjugates of DNA and cells by linking the DNA to a native functional group on the cell surface. The cells can be without cell walls or can have cell walls. The modified cells can be linked to a substrate surface and used in assay or bioreactors.
-
Wu, Xianai; Lehmler, Hans-Joachim
2015-01-01
Chiral polychlorinated biphenyl (PCB) congeners, such as PCB 136, are atropselectively metabolized to various hydroxylated PCB metabolites (HO-PCBs). The present study investigates the effect of two thiol antioxidants, glutathione and N-acetyl-cysteine (NAC), on profiles and chiral signatures of PCB 136 and its HO-PCB metabolites in rat liver microsomal incubations. Liver microsomes prepared from rats pretreated with phenobarbital were incubated with PCB 136 (5 μM) in the presence of the respective antioxidant (0–10 mM), and levels and chiral signatures of PCB 136 and its HO-PCB metabolites were determined. Three metabolites, 5-136 (2,2′,3,3′,6,6′-hexachlorobiphenyl-5-ol), 4-136 (2,2′,3,3′,6,6′-hexachlorobiphenyl-4-ol) and 4,5-136 (2,2′,3,3′,6,6′-hexachlorobiphenyl-4,5-diol), were detected in all incubations, with 5-136 being the major metabolite. Compared to microsomal incubations without antioxidant, levels of 4,5-136 increased with increasing antioxidant concentration, whereas levels of PCB 136 and both mono-HO-PCBs were not affected by the presence of either antioxidant. PCB 136, 4-136 and 5-136 displayed significant atropisomeric enrichment; however, the direction and extent of the atropisomeric enrichment was not altered in the presence of an antioxidant. Because 4,5-136 can either be conjugated to a sulfate or glucuronide metabolite that is readily excreted or further oxidized a potentially toxic PCB 136 quinone, the effect of both thiol antioxidants on 4,5-136 formation suggests that disruptions of glutathione homeostasis may alter the balance between both metabolic pathways and, thus, PCB 136 toxicity in vivo. PMID:26155892
-
Structure and function of nanoparticle-protein conjugates
International Nuclear Information System (INIS)
Aubin-Tam, M-E; Hamad-Schifferli, K
2008-01-01
Conjugation of proteins to nanoparticles has numerous applications in sensing, imaging, delivery, catalysis, therapy and control of protein structure and activity. Therefore, characterizing the nanoparticle-protein interface is of great importance. A variety of covalent and non-covalent linking chemistries have been reported for nanoparticle attachment. Site-specific labeling is desirable in order to control the protein orientation on the nanoparticle, which is crucial in many applications such as fluorescence resonance energy transfer. We evaluate methods for successful site-specific attachment. Typically, a specific protein residue is linked directly to the nanoparticle core or to the ligand. As conjugation often affects the protein structure and function, techniques to probe structure and activity are assessed. We also examine how molecular dynamics simulations of conjugates would complete those experimental techniques in order to provide atomistic details on the effect of nanoparticle attachment. Characterization studies of nanoparticle-protein complexes show that the structure and function are influenced by the chemistry of the nanoparticle ligand, the nanoparticle size, the nanoparticle material, the stoichiometry of the conjugates, the labeling site on the protein and the nature of the linkage (covalent versus non-covalent)
-
The Synthesis of Substituted Piperazine-cholesterol Conjugates for ...
African Journals Online (AJOL)
A small library of cholesterol-piperazine conjugates were synthesized by the reaction of cholesteryl chloroformate with a set of substituted piperazines in dichloromethane at room temperature. The conjugates, all obtained in good to excellent yields, were synthesized to be key components of nucleic acid transfection ...
-
Nanostructured conjugated polymers in chemical sensors: synthesis, properties and applications.
Correa, D S; Medeiros, E S; Oliveira, J E; Paterno, L G; Mattoso, Luiz C
2014-09-01
Conjugated polymers are organic materials endowed with a π-electron conjugation along the polymer backbone that present appealing electrical and optical properties for technological applications. By using conjugated polymeric materials in the nanoscale, such properties can be further enhanced. In addition, the use of nanostructured materials makes possible miniaturize devices at the micro/nano scale. The applications of conjugated nanostructured polymers include sensors, actuators, flexible displays, discrete electronic devices, and smart fabric, to name a few. In particular, the use of conjugated polymers in chemical and biological sensors is made feasible owning to their sensitivity to the physicochemical conditions of its surrounding environment, such as chemical composition, pH, dielectric constant, humidity or even temperature. Subtle changes in these conditions bring about variations on the electrical (resistivity and capacitance), optical (absorptivity, luminescence, etc.), and mechanical properties of the conjugated polymer, which can be precisely measured by different experimental methods and ultimately associated with a specific analyte and its concentration. The present review article highlights the main features of conjugated polymers that make them suitable for chemical sensors. An especial emphasis is given to nanostructured sensors systems, which present high sensitivity and selectivity, and find application in beverage and food quality control, pharmaceutical industries, medical diagnosis, environmental monitoring, and homeland security, and other applications as discussed throughout this review.
-
Antioxidants in bakery products: a review.
Nanditha, B; Prabhasankar, P
2009-01-01
Fats impart taste and texture to the product but it is susceptible to oxidation leading to the development of rancidity and off-flavor. Since ancient times it has been in practice to use antioxidants in foods. Discovery of synthetic antioxidants has revolutionized the use of antioxidants in food. The effect of these antioxidants in bakery products were reviewed and found to be effective in enhancing the shelf life. Animal experimental studies have shown that some of the synthetic antioxidants had toxigenic, mutagenic, and carcinogenic effects. Hence there is an increasing demand for the use of natural antioxidants in foods, especially in bakery products. Some of the natural antioxidants such as alpha-tocopherol, beta-carotene, and ascorbic acid were already used in bakery products. These natural antioxidants are found to be effective in enhancing the shelf life of bakery products but not to the extent of synthetic antioxidants. Baking processing steps may lower the antioxidative activity but techniques such as encapsulation of antioxidants can retain their activity. Antioxidative activity of the plant extracts such as garcinia, curcumin, vanillins, and mint were reviewed but studies on their role in bakery products were limited or very few. Hence there is a wide scope for study under this direction in depth.
-
Intense correlation between brain infarction and protein-conjugated acrolein.
Saiki, Ryotaro; Nishimura, Kazuhiro; Ishii, Itsuko; Omura, Tomohiro; Okuyama, Shigeru; Kashiwagi, Keiko; Igarashi, Kazuei
2009-10-01
We recently found that increases in plasma levels of protein-conjugated acrolein and polyamine oxidases, enzymes that produce acrolein, are good markers for stroke. The aim of this study was to determine whether the level of protein-conjugated acrolein is increased and levels of spermine and spermidine, the substrates of acrolein production, are decreased at the locus of infarction. A unilateral infarction was induced in mouse brain by photoinduction after injection of Rose Bengal. The volume of the infarction was analyzed using the public domain National Institutes of Health image program. The level of protein-conjugated acrolein at the locus of infarction and in plasma was measured by Western blotting and enzyme-linked immunosorbent assay, respectively. The levels of polyamines at the locus of infarction and in plasma were measured by high-performance liquid chromatography. The level of protein-conjugated acrolein was greatly increased, and levels of spermine and spermidine were decreased at the locus of infarction at 24 hours after the induction of stroke. The size of infarction was significantly decreased by N-acetylcysteine, a scavenger of acrolein. It was also found that the increases in the protein-conjugated acrolein, polyamines, and polyamine oxidases in plasma were observed after the induction of stroke. The results indicate that the induction of infarction is well correlated with the increase in protein-conjugated acrolein at the locus of infarction and in plasma.
-
Optical observations geomagnetically conjugate to sprite-producing lightning discharges
Directory of Open Access Journals (Sweden)
R. A. Marshall
2005-09-01
Full Text Available Theoretical studies have predicted that large positive cloud-to-ground discharges can trigger a runaway avalanche process of relativistic electrons, forming a geomagnetically trapped electron beam. The beam may undergo pitch angle and energy scattering during its traverse of the Earth's magnetosphere, with a small percentage of electrons remaining in the loss cone and precipitating in the magnetically conjugate atmosphere. In particular, N2 1P and N2+1N optical emissions are expected to be observable. In July and August 2003, an attempt was made to detect these optical emissions, called "conjugate sprites", in correlation with sprite observations in Europe near . Sprite observations were made from the Observatoire du Pic du Midi (OMP in the French Pyrenées, and VLF receivers were installed in Europe to detect causative sferics and ionospheric disturbances associated with sprites. In the Southern Hemisphere conjugate region, the Wide-angle Array for Sprite Photometry (WASP was deployed at the South African Astronomical Observatory (SAAO, near Sutherland, South Africa, to observe optical emissions with a field-of-view magnetically conjugate to the Northern Hemisphere observing region. Observations at OMP revealed over 130 documented sprites, with WASP observations covering the conjugate region successfully for 30 of these events. However, no incidences of optical emissions in the conjugate hemisphere were found. Analysis of the conjugate optical data from SAAO, along with ELF energy measurements from Palmer Station, Antarctica, and charge-moment analysis, show that the lightning events during the course of this experiment likely had insufficient intensity to create a relativistic beam. Keywords. Ionosphere (Ionsophere-magnetosphere interactions; Ionospheric disturbances; Instruments and techniques
-
Minimizing inner product data dependencies in conjugate gradient iteration
Vanrosendale, J.
1983-01-01
The amount of concurrency available in conjugate gradient iteration is limited by the summations required in the inner product computations. The inner product of two vectors of length N requires time c log(N), if N or more processors are available. This paper describes an algebraic restructuring of the conjugate gradient algorithm which minimizes data dependencies due to inner product calculations. After an initial start up, the new algorithm can perform a conjugate gradient iteration in time c*log(log(N)).
-
Covalently bound conjugates of albumin and heparin: Synthesis, fractionation and characterization
Hennink, Wim E.; Feijen, Jan; Ebert, Charles D.; Kim, Sung Wan
1983-01-01
Covalently bound conjugates of human serum albumin and heparin were prepared as compounds which could improve the blood-compatibility of polymer surfaces either by preadsorption or by covalent coupling of the conjugates onto blood contacting surfaces. The conjugates (10–16 weight % of heparin) were
-
Directory of Open Access Journals (Sweden)
Ming Yuan
2014-01-01
Full Text Available Hawk tea (Litsea coreana var. lanuginose is a very popular herbal tea in the southwest of China. According to the maturity degree of raw materials, Hawk tea can usually be divided into three types: Hawk bud tea (HB, Hawk primary leaf tea (HP, and Hawk mature leaf tea (HM. In this study, some of the bioactive constituents and antioxidant properties of the three kinds of Hawk tea infusions were comparatively investigated. The results showed that the contents of total flavonoids, vitamin C, and carbohydrates in Hawk bud tea infusion (HBI were higher than those in Hawk primary leaf tea infusion (HPI and Hawk mature leaf tea infusion (HMI. HPI had higher contents of total polyphenols and exhibited better DPPH radical scavenging activity and ferric reducing activity power. HBI could provide more effective protection against erythrocyte hemolysis. As age is going from bud to mature leaf, the ability to inhibit the formation of low density lipoprotein (LDL conjugated diene and the loss of tryptophan fluorescence decreased. The bioactive constituents and antioxidant activities of Hawk tea infusions were significantly affected by the maturity degree of the raw material.
-
Small angle scattering from protein/sugar conjugates
International Nuclear Information System (INIS)
Jackson, Andrew; White, John
2006-01-01
The Maillard reaction between free amine groups on proteins and sugars is well known. We have examined the effect of the reaction of the casein group of milk proteins with sugars on their nanoscale structure and aggregation. The small angle neutron scattering from beta casein and sodium caseinate and their sugar conjugates have been studied as a function of solution concentration. At high conjugate concentration (greater than ca. 5mg/ml) the addition of sugar reduces supra-micellar aggregation of the protein whilst at lower concentration, where the protein is expected to be deaggregated already, little effect is seen. Guinier analysis of the scattering data show a radius of gyration of around 75A-bar for beta casein in solution and around 80A-bar for the sucrose conjugate
-
Small angle scattering from protein/sugar conjugates
Energy Technology Data Exchange (ETDEWEB)
Jackson, Andrew [Research School of Chemistry, Australian National University, Canberra, ACT 0200 (Australia)]. E-mail: ajj@nist.gov; White, John [Research School of Chemistry, Australian National University, Canberra, ACT 0200 (Australia)
2006-11-15
The Maillard reaction between free amine groups on proteins and sugars is well known. We have examined the effect of the reaction of the casein group of milk proteins with sugars on their nanoscale structure and aggregation. The small angle neutron scattering from beta casein and sodium caseinate and their sugar conjugates have been studied as a function of solution concentration. At high conjugate concentration (greater than ca. 5mg/ml) the addition of sugar reduces supra-micellar aggregation of the protein whilst at lower concentration, where the protein is expected to be deaggregated already, little effect is seen. Guinier analysis of the scattering data show a radius of gyration of around 75A-bar for beta casein in solution and around 80A-bar for the sucrose conjugate.
-
Deciphering conjugative plasmid permissiveness in wastewater microbiomes
DEFF Research Database (Denmark)
Jacquiod, Samuel Jehan Auguste; Brejnrod, Asker Daniel; Milani, Stefan Morberg
2017-01-01
Wastewater treatment plants (WWTPs) are designed to robustly treat polluted water. They are characterized by ceaseless flows of organic, chemical and microbial matter, followed by treatment steps before environmental release. WWTPs are hotspots of horizontal gene transfer between bacteria via...... still remains largely uncharted. Furthermore, current in vitro methods used to assess conjugation in complex microbiomes do not include in situ behaviours of recipient cells, resulting in partial understanding of transfers. We investigated the in vitro conjugation capacities of WWTP microbiomes from...... inlet sewage and outlet treated water using the broad-host range IncP-1 conjugative plasmid, pKJK5. A thorough molecular approach coupling metagenomes to 16S rRNA DNA/cDNA amplicon sequencing was established to characterize microbiomes using the ecological concept of functional response groups. A broad...
-
Cheewatanakornkool, Kamonrak; Niratisai, Sathit; Manchun, Somkamol; Dass, Crispin R; Sriamornsak, Pornsak
2017-10-15
In this paper, pectin was cross-linked by a coupling reaction with either thioglycolic acid or cystamine dihydrochloride to form thiolated pectins. The thiolated pectins were then coupled with doxorubicin (DOX) derivative to obtain thiolated pectin-DOX conjugates by two different methods, disulfide bond formation and disulfide bond exchange. The disulfide bond exchange method provided a simple, fast, and efficient approach for synthesis of thiolated pectin-DOX conjugates, compared to the disulfide bond formation. Characteristics, physicochemical properties, and morphology of thiolated pectins and thiolated pectin-DOX conjugates were determined. DOX content in thiolated pectin-DOX conjugates using low methoxy pectin was found to be higher than that using high methoxy pectin. The in vitro anticancer activity of thiolated pectin-DOX conjugates was significantly higher than that of free DOX, in mouse colon carcinoma and human bone osteosarcoma cells, but insignificantly different from that of free DOX, in human prostate cancer cells. Due to their promising anticancer activity in mouse colon carcinoma cells, the thiolated pectin-DOX conjugates might be suitable for building drug platform for colorectal cancer-targeted delivery of DOX. Copyright © 2017 Elsevier Ltd. All rights reserved.
-
Less is More: A Comparison of Antibody-Gold Nanoparticle Conjugates of Different Ratios.
Byzova, Nadezhda A; Safenkova, Irina V; Slutskaya, Elvira S; Zherdev, Anatoly V; Dzantiev, Boris B
2017-11-15
This comprehensive study is related to gold nanoparticles (GNPs) conjugated with antibodies. The goal of the study is to determine the minimal concentration of antibodies for conjugate synthesis when the conjugates have high antigen-capturing activity. Two systems were studied: gold nanoparticles conjugated with monoclonal antibodies (mAb-GNP) specific to Helicobacter pylori and gold nanoparticles conjugated with polyclonal antibodies (pAb-GNP) specific to mouse immunoglobulins. Several conjugates were synthesized with different GNP-to-antibody molar ratios (from 1:1 to 1:245) through nondirectional and noncovalent immobilization on a surface of GNPs with a diameter of 25.3 ± 4.6 nm. The maximal antigen-capturing activities and equilibrium constants of the conjugates correlate with the formation of a constant hydrodynamic radius of the conjugates for mAb-GNP (GNP to antibody molar ratio 1:58) and with the stabilizing concentration by flocculation curves for pAb-GNP (GNP to antibody molar ratio 1:116). The application of the conjugates to the lateral flow immunoassay shows that the antibody concentrations used for the conjugation can be reduced (below the stabilizing concentration) without losing activity for the mAb-GNP conjugates. The findings highlight that the optimal concentration of antibodies immobilized on the surface of GNPs is not always equal to the stabilizing concentration determined by the flocculation curve.
-
Conjugation vs hyperconjugation in molecular structure of acrolein
Shishkina, Svitlana V.; Slabko, Anzhelika I.; Shishkin, Oleg V.
2013-01-01
Analysis of geometric parameters of butadiene and acrolein reveals the contradiction between the Csp2-Csp2 bond length in acrolein and classical concept of conjugation degree in the polarized molecules. In this Letter the reasons of this contradiction have been investigated. It is concluded that the Csp2-Csp2 bond length in acrolein is determined by influence of the bonding for it π-π conjugation and antibonding n → σ∗ hyperconjugation between the oxygen lone pair and the antibonding orbital of the single bond. It was shown also this bond length depends on the difference in energy of conjugative and hyperconjugative interactions.
-
Enhanced photodynamic efficacy of zinc phthalocyanine by conjugating to heptalysine.
Li, Linsen; Luo, Zhipu; Chen, Zhuo; Chen, Jincan; Zhou, Shanyong; Xu, Peng; Hu, Ping; Wang, Jundong; Chen, Naisheng; Huang, Jinling; Huang, Mingdong
2012-11-21
Zinc phthalocyanine (ZnPc) is a promising photosensitizer for photodynamic therapy, but faces some challenges: ZnPc is insoluble in water and thus requires either special formulation of ZnPc by, e.g., liposome or Cremophor EL, or chemical modification of Pc ring to enhance its bioavailability and photodynamic efficacy. Here, we conjugated monosubstituted ZnPc-COOH with a series of oligolysine moieties with different numbers of lysine residues (ZnPc-(Lys)(n) (n = 1, 3, 5, 7, 9) to improve the water solubility of the ZnPc conjugates. We measured the photosensitizing efficacies and the cellular uptakes of this series of conjugates on a normal and a cancerous cell line. In addition, we developed a sensitive in situ method to distinguish the difference in photodynamic efficacy among conjugates. Our results showed that ZnPc-(Lys)(7) has the highest photodynamic efficacy compared to the other conjugates investigated.
-
International Nuclear Information System (INIS)
Nguyen Quoc Hien; Nguyen Van Toan; Vo Tan Thien; Le Hai
2000-01-01
The thermo-oxidative aging resistance of radiation vulcanization of natural rubber latex (RVNRL) products should be adequately by using suitable antioxidants or new kind of effective antioxidant. This work presents the results of preparation of natural antioxidant from hair keratin. Characteristics and effectiveness of resultant antioxidant are also presented. The results obtained indicates that antioxidant made from hair keratin is safe and effective for rubber products from RVNRL. (author)
-
Application of the conjugate-gradient method to ground-water models
Manteuffel, T.A.; Grove, D.B.; Konikow, Leonard F.
1984-01-01
The conjugate-gradient method can solve efficiently and accurately finite-difference approximations to the ground-water flow equation. An aquifer-simulation model using the conjugate-gradient method was applied to a problem of ground-water flow in an alluvial aquifer at the Rocky Mountain Arsenal, Denver, Colorado. For this application, the accuracy and efficiency of the conjugate-gradient method compared favorably with other available methods for steady-state flow. However, its efficiency relative to other available methods depends on the nature of the specific problem. The main advantage of the conjugate-gradient method is that it does not require the use of iteration parameters, thereby eliminating this partly subjective procedure. (USGS)
-
Nonlinear propagation of phase-conjugate focused sound beams in water
Brysev, A. P.; Krutyansky, L. M.; Preobrazhensky, V. L.; Pyl'nov, Yu. V.; Cunningham, K. B.; Hamilton, M. F.
2000-07-01
Nonlinear propagation of phase-conjugate, focused, ultrasound beams is studied. Measurements are presented of harmonic amplitudes along the axis and in the focal plane of the conjugate beam, and of the waveform and spectrum at the focus. A maximum peak pressure of 3.9 MPa was recorded in the conjugate beam. The measurements are compared with simulations based on the KZK equation, and satisfactory agreement is obtained.
-
Repenko, Tatjana; Rix, Anne; Ludwanowski, Simon; Go, Dennis; Kiessling, Fabian; Lederle, Wiltrud; Kuehne, Alexander J C
2017-09-07
Conjugated polymer nanoparticles exhibit strong fluorescence and have been applied for biological fluorescence imaging in cell culture and in small animals. However, conjugated polymer particles are hydrophobic and often chemically inert materials with diameters ranging from below 50 nm to several microns. As such, conjugated polymer nanoparticles cannot be excreted through the renal system. This drawback has prevented their application for clinical bio-medical imaging. Here, we present fully conjugated polymer nanoparticles based on imidazole units. These nanoparticles can be bio-degraded by activated macrophages. Reactive oxygen species induce scission of the conjugated polymer backbone at the imidazole unit, leading to complete decomposition of the particles into soluble low molecular weight fragments. Furthermore, the nanoparticles can be surface functionalized for directed targeting. The approach opens a wide range of opportunities for conjugated polymer particles in the fields of medical imaging, drug-delivery, and theranostics.Conjugated polymer nanoparticles have been applied for biological fluorescence imaging in cell culture and in small animals, but cannot readily be excreted through the renal system. Here the authors show fully conjugated polymer nanoparticles based on imidazole units that can be bio-degraded by activated macrophages.
-
Oxidant-Antioxidant Balance in Severe Brain Injury
Directory of Open Access Journals (Sweden)
N. N. Yepifantseva
2010-01-01
Full Text Available Objective: to study the time course of changes in oxidative status parameters and their relationship with inflammation mediators in the acute period of severe brain injury (SBI. Subjects and methods. One hundred and thirteen patients aged 17—67 years were examined. The injury was closed and open in 54 (47.8% and 59 (52.2% patients, respectively. Severe brain contusions were observed in 47 patients, diffuse axonal lesions were seen in 2, and intracranial hematomas were present in 64 patients. The Glasgow coma scores for admission consciousness loss were 6.8±0.25. A control group comprised 23 healthy individuals. The significance of differences was estimated by Student’s test, Wilcoxon-Mann-Whitney, test, Spearman’s correlation test. Venous blood samples were used to study total oxidative activity (TOA and total antioxidative activity (TAA, diene conjugates, lactic acid, albumin, transferrin (TF, ceruloplasmin, C-reactive protein, and lactoferrin (LF were measured in venous blood on disease days 1, 4, 7, 10, 14, and 21. The profile of plasma cytokines (IL-1j8, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, TNF-а, and IFN-y was studied by flow fluorometry on a Cytomics FC 500 cytofluorometer (Beckman Counlter, USA (reagents were from Bender Medsystems, Austria. Results. In SBI, there was an increase in oxidants, a reduction in antioxidant activity, and lipid peroxidation activation, which were closely related. The oxidation coefficient (TOA/TAA was 40 times greater than the normal values on days 7 to 10. The oxidation parameters were found to be associated with inflammation and cytokine-mediated immunological reactions. The time course of changes in the study proteins was characteristic for systemic inflammation and there was an association with oxidative processes only for ceruloplasm. TF was found to have an association with IL-5 and IL-10, which reflects its involvement in immunological reactions. The association with hypoxia was
-
Mao, Shuqin; Wang, Kaidi; Lei, Yukun; Yao, Shuting; Lu, Baiyi; Huang, Weisu
2017-01-01
The antioxidant synergistic effects of Osmanthus fragrans flowers with green tea were evaluated, and their major antioxidant compounds contributed to the total amount of synergy were determined. The antioxidant compounds in O. fragrans flowers with green tea were identified by LC-MS and quantified by UPLC-PDA. The synergistic antioxidant interactions between O. fragrans flowers with green tea and their antioxidant compounds were tested using the Prieto?s model after the simulated digestion. T...
-
Yang, Tsung-Shi; Liu, Tai-Ti; Lin, I-Hwa
2017-08-01
The aims of this research were to conjugate chitosan (CT) with stearic acid (SA) and gallic acid (GA), and apply the modified chitosan to stabilize labile aroma compounds such as allyl isothiocyanate (AITC) and limonene in oil-in-water emulsions. Generally, the antioxidant activity of CT-SA-GA increased as the GA content in the conjugate increased. In most assays, GA had a lower IC 50 value than that of CT-SA-GA; however, CT-SA-GA exhibited better performance than GA in the Fe 2+ -chelating activity. In accelerated tests (heating or illumination) for evaluating the chemical stability of AITC and limonene during storage, CT-SA and CT-SA-GA were used to prepare AITC and limonene O/W emulsions, respectively. Tween 80 and Span 80 (T-S-80), an emulsifier mixture, were used as a control in both emulsions for comparison. The results show that CT-SA or CT-SA-GA could protect AITC or limonene from degradation or oxidation more effectively than T-S-80. Copyright © 2017 Elsevier Ltd. All rights reserved.
-
Optical observations geomagnetically conjugate to sprite-producing lightning discharges
Directory of Open Access Journals (Sweden)
R. A. Marshall
2005-09-01
Full Text Available Theoretical studies have predicted that large positive cloud-to-ground discharges can trigger a runaway avalanche process of relativistic electrons, forming a geomagnetically trapped electron beam. The beam may undergo pitch angle and energy scattering during its traverse of the Earth's magnetosphere, with a small percentage of electrons remaining in the loss cone and precipitating in the magnetically conjugate atmosphere. In particular, N2 1P and N2+1N optical emissions are expected to be observable. In July and August 2003, an attempt was made to detect these optical emissions, called "conjugate sprites", in correlation with sprite observations in Europe near
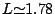 . Sprite observations were made from the Observatoire du Pic du Midi (OMP in the French Pyrenées, and VLF receivers were installed in Europe to detect causative sferics and ionospheric disturbances associated with sprites. In the Southern Hemisphere conjugate region, the Wide-angle Array for Sprite Photometry (WASP was deployed at the South African Astronomical Observatory (SAAO, near Sutherland, South Africa, to observe optical emissions with a field-of-view magnetically conjugate to the Northern Hemisphere observing region. Observations at OMP revealed over 130 documented sprites, with WASP observations covering the conjugate region successfully for 30 of these events. However, no incidences of optical emissions in the conjugate hemisphere were found. Analysis of the conjugate optical data from SAAO, along with ELF energy measurements from Palmer Station, Antarctica, and charge-moment analysis, show that the lightning events during the course of this experiment likely had insufficient intensity to create a relativistic beam.
. Sprite observations were made from the Observatoire du Pic du Midi (OMP in the French Pyrenées, and VLF receivers were installed in Europe to detect causative sferics and ionospheric disturbances associated with sprites. In the Southern Hemisphere conjugate region, the Wide-angle Array for Sprite Photometry (WASP was deployed at the South African Astronomical Observatory (SAAO, near Sutherland, South Africa, to observe optical emissions with a field-of-view magnetically conjugate to the Northern Hemisphere observing region. Observations at OMP revealed over 130 documented sprites, with WASP observations covering the conjugate region successfully for 30 of these events. However, no incidences of optical emissions in the conjugate hemisphere were found. Analysis of the conjugate optical data from SAAO, along with ELF energy measurements from Palmer Station, Antarctica, and charge-moment analysis, show that the lightning events during the course of this experiment likely had insufficient intensity to create a relativistic beam.
Keywords. Ionosphere (Ionsophere-magnetosphere interactions; Ionospheric disturbances; Instruments and techniques
-
Bromberg, Lev; Raduyk, Svetlana; Hatton, T Alan; Concheiro, Angel; Rodriguez-Valencia, Cosme; Silva, Maite; Alvarez-Lorenzo, Carmen
2009-05-20
Conjugates of linear and branched polyethyleneimine (PEI) and monoamine polyether Jeffamine M-2070 (PO/EO mol ratio 10/31, 2000 Da) were synthesized through polyether activation by cyanuric chloride followed by attachment to PEI and guanidinylation by 1H-pyrazole-carboxamidine hydrochloride. The resulting guanidinylated PEI-polyether conjugates (termed gPEI-Jeffamine) efficiently complexed plasmid DNA, and their polyplexes possessed enhanced colloidal stability in the presence of serum proteins. In vitro studies with mammalian CHO-1, 3T3, and Cos-7 cell lines demonstrated improved transfection efficiency of the pCMVbeta-gal plasmid/gPEI-Jeffamine polyplexes. The guanidinylation of the amino groups of PEI and the conjugation of PEI with the Jeffamine polyether enhanced the conjugates' interaction with genetic material and reduced the cytotoxicity of the polyplexes in experiments with the L929 cell line.
-
Joana Aguiar; Berta Nogueiro Estevinho; Lúcia Silveira Santos
2016-01-01
Background: Functional foods fortified with antioxidants are gaining more popularity since consumption alone of foods naturally rich in antioxidants is insufficient to reduce oxidative stress associated with various diseases. Despite their beneficial effects, natural antioxidants present in coffee are sensitive to heat, light and oxygen, limiting their application in the food industry. Although microencapsulation is able to protect the antioxidant from degradation, mask its taste and control ...
-
DEFF Research Database (Denmark)
Møller, Thea S B; Liu, Gang; Boysen, Anders
2017-01-01
research suggests that the effect of antibiotic treatment on plasmid conjugation frequencies, and hence the spread of resistance plasmids, may have been overestimated. We addressed the question by quantifying transfer proteins and conjugation frequencies of a blaCTX-M-1 encoding IncI1 resistance plasmid....... The frequency of plasmid conjugation, measured in an antibiotic free environment, increased significantly when the donor was pre-grown in broth containing CTX compared to growth without this drug, regardless of whether blaCTX-M-1 was located on the plasmid or in trans on the chromosome. The results shows...
-
125I Radioimmunoassay of serum ursodeoxycholyl conjugates
International Nuclear Information System (INIS)
Hill, A.; Ross, P.E.; Bouchier, I.A.D.
1983-01-01
A radioimmunoassay for serum ursodeoxycholic conjugates using an iodine-125 ligand has been developed. The bile acid was present in normal fasting serum (0.19 +- SD 0.19 μmol/l, n=24) and 2-hour post-prandial serum (0.8 +- SD 0.8 μmol/l, n=16). Gallstone patients undergoing oral ursodeoxycholic acid therapy had significantly higher post-prandial serum levels (21.5 +- SD 14.0 μmol/l, n=15) by radioimmunoassay. Gas liquid chromatography analysis indicated that in normal serum ursodeoxycholic acid was totally conjugated, whereas sera from gallstone patients contained a proportion as the free bile acid (10.2 +- SD 8.1 μmol/l, n=15). Following an oral dose of ursodeoxycholic acid, both unconjugated and conjugated forms of the bile acid appeared in the serum of healthy individuals. (Auth.)
-
Scintillation Reduction using Conjugate-Plane Imaging (Abstract)
Vander Haagen, G. A.
2017-12-01
(Abstract only) All observatories are plagued by atmospheric turbulence exhibited as star scintillation or "twinkle" whether a high altitude adaptive optics research or a 30-cm amateur telescope. It is well known that these disturbances are caused by wind and temperature-driven refractive gradients in the atmosphere and limit the ultimate photometric resolution of land-based facilities. One approach identified by Fuchs (1998) for scintillation noise reduction was to create a conjugate image space at the telescope and focus on the dominant conjugate turbulent layer within that space. When focused on the turbulent layer little or no scintillation exists. This technique is described whereby noise reductions of 6 to 11/1 have been experienced with mathematical and optical bench simulations. Discussed is a proof-of-principle conjugate optical train design for an 80-mm, f7 telescope.
-
Bispecific small molecule-antibody conjugate targeting prostate cancer.
Kim, Chan Hyuk; Axup, Jun Y; Lawson, Brian R; Yun, Hwayoung; Tardif, Virginie; Choi, Sei Hyun; Zhou, Quan; Dubrovska, Anna; Biroc, Sandra L; Marsden, Robin; Pinstaff, Jason; Smider, Vaughn V; Schultz, Peter G
2013-10-29
Bispecific antibodies, which simultaneously target CD3 on T cells and tumor-associated antigens to recruit cytotoxic T cells to cancer cells, are a promising new approach to the treatment of hormone-refractory prostate cancer. Here we report a site-specific, semisynthetic method for the production of bispecific antibody-like therapeutics in which a derivative of the prostate-specific membrane antigen-binding small molecule DUPA was selectively conjugated to a mutant αCD3 Fab containing the unnatural amino acid, p-acetylphenylalanine, at a defined site. Homogeneous conjugates were generated in excellent yields and had good solubility. The efficacy of the conjugate was optimized by modifying the linker structure, relative binding orientation, and stoichiometry of the ligand. The optimized conjugate showed potent and selective in vitro activity (EC50 ~ 100 pM), good serum half-life, and potent in vivo activity in prophylactic and treatment xenograft mouse models. This semisynthetic approach is likely to be applicable to the generation of additional bispecific agents using drug-like ligands selective for other cell-surface receptors.
-
Bispecific small molecule–antibody conjugate targeting prostate cancer
Kim, Chan Hyuk; Axup, Jun Y.; Lawson, Brian R.; Yun, Hwayoung; Tardif, Virginie; Choi, Sei Hyun; Zhou, Quan; Dubrovska, Anna; Biroc, Sandra L.; Marsden, Robin; Pinstaff, Jason; Smider, Vaughn V.; Schultz, Peter G.
2013-01-01
Bispecific antibodies, which simultaneously target CD3 on T cells and tumor-associated antigens to recruit cytotoxic T cells to cancer cells, are a promising new approach to the treatment of hormone-refractory prostate cancer. Here we report a site-specific, semisynthetic method for the production of bispecific antibody-like therapeutics in which a derivative of the prostate-specific membrane antigen-binding small molecule DUPA was selectively conjugated to a mutant αCD3 Fab containing the unnatural amino acid, p-acetylphenylalanine, at a defined site. Homogeneous conjugates were generated in excellent yields and had good solubility. The efficacy of the conjugate was optimized by modifying the linker structure, relative binding orientation, and stoichiometry of the ligand. The optimized conjugate showed potent and selective in vitro activity (EC50 ∼100 pM), good serum half-life, and potent in vivo activity in prophylactic and treatment xenograft mouse models. This semisynthetic approach is likely to be applicable to the generation of additional bispecific agents using drug-like ligands selective for other cell-surface receptors. PMID:24127589
-
Synthesis and characterization of nido-carborane-cobalamin conjugates
International Nuclear Information System (INIS)
Hogenkamp, Harry P.C.; Collins, Douglas A.; Live, David; Benson, Linda M.; Naylor, Stephen
2000-01-01
Three vitamin B 12 (cyanocobalamin) conjugates bearing one nido-carborane molecule or two nido-carborane molecules linked to the propionamide side chains via a four carbon linker have been synthesized. Reaction of o-carboranoylchloride with 1,4-diaminobutane in pyridine produced nido-carboranoyl(4-amidobutyl)amine, which was linked to the b- and d-monocarboxylic acids and the b,d-dicarboxylic acid of cyanocobalamin. Mass spectrometry analysis as well as 11 B nuclear magnetic resonance demonstrated that during the reaction of o-carboranonylchloride with diaminobutane one of the boron atoms was eliminated. In vitro biological activity of the cyanocobalamin-nido-carborane conjugates was assessed by the unsaturated vitamin B 12 binding capacity assay. When compared with 57 Co cyanocobalamin, the biological activity of cyanocobalamin-b-nido-carborane, cyanocobalamin-d-nido-carborane, and cyanocobalamin-b-d-bis-nido-carborane conjugates were 92.93%, 35.75%, and 37.02%, respectively. These findings suggest that the 10 B cobalamin conjugates might be useful agents in treating malignant tumors via neutron capture therapy
-
Shahnaz, G; Perera, G; Sakloetsakun, D; Rahmat, D; Bernkop-Schnürch, A
2010-05-21
This study was aimed at improving the mucoadhesive properties of carboxymethyl dextran by the covalent attachment of cysteine. Mediated by a carbodiimide, l-cysteine was covalently attached to the polymer. The resulting CMD-cysteine conjugate (CMD-(273) conjugate) displayed 273+/-20 micromol thiol groups per gram of polymer (mean+/-S.D.; n=3). Within 2h the viscosity of an aqueous mucus/CMD-(273) conjugate mixture pH 7.4 increased at 37 degrees C by more than 85% compared to a mucus/carboxymethyl dextran mixture indicating enlarged interactions between the mucus and the thiolated polymer. Due to the immobilization of cysteine, the swelling velocity of the polymer was significantly accelerated (ppolymer disintegrated within 15 min, whereas tablets of the CMD-(273) conjugate remained stable for 160 min (means+/-S.D.; n=3). Results from LDH and MTT assays on Caco-2 cells revealed 4.96+/-0.98% cytotoxicity and 94.1+/-0.9% cell viability for the CMD-(273) conjugate, respectively. Controlled release of model compound from CMD-(273) conjugate tablets was observed over 6h. These findings suggest that CMD-(273) conjugate is a promising novel polymer for drug delivery systems providing improved mucoadhesive and cohesive properties, greater stability and biocompatibility. Copyright 2010 Elsevier B.V. All rights reserved.
-
Structure Property Relationships in Organic Conjugated Systems
O'Neill, Luke; Lynch, Patrick; McNamara, Mary
2005-01-01
A series of π conjugated oligomers were studied by absorption and photoluminescence spectroscopy. A linear relationship between the positioning of the absorption and photoluminescence maxima plotted against inverse conjugation length is observed. The relationships are in good agreement with the simple particle in a box method, one of the earliest descriptions of the properties of one-dimensional organic molecules. In addition to the electronic transition energies, it was observed that the Sto...
-
Protein carriers of conjugate vaccines: characteristics, development, and clinical trials.
Pichichero, Michael E
2013-12-01
The immunogenicity of polysaccharides as human vaccines was enhanced by coupling to protein carriers. Conjugation transformed the T cell-independent polysaccharide vaccines of the past to T cell-dependent antigenic vaccines that were much more immunogenic and launched a renaissance in vaccinology. This review discusses the conjugate vaccines for prevention of infections caused by Hemophilus influenzae type b, Streptococcus pneumoniae, and Neisseria meningitidis. Specifically, the characteristics of the proteins used in the construction of the vaccines including CRM, tetanus toxoid, diphtheria toxoid, Neisseria meningitidis outer membrane complex, and Hemophilus influenzae protein D are discussed. The studies that established differences among and key features of conjugate vaccines including immunologic memory induction, reduction of nasopharyngeal colonization and herd immunity, and antibody avidity and avidity maturation are presented. Studies of dose, schedule, response to boosters, of single protein carriers with single and multiple polysaccharides, of multiple protein carriers with multiple polysaccharides and conjugate vaccines administered concurrently with other vaccines are discussed along with undesirable consequences of conjugate vaccines. The clear benefits of conjugate vaccines in improving the protective responses of the immature immune systems of young infants and the senescent immune systems of the elderly have been made clear and opened the way to development of additional vaccines using this technology for future vaccine products.
-
Gubskiy, Iu I; Paramonova, G I; Boldeskul, A E; Primak, R G; Bogdanova, L A; Zadorina, O V; Litvinova, N V
1992-01-01
Lipid peroxidation (LPO), physico-chemical properties of the membranes and isoformic composition of microsomal cytochrome P-450 from the rat liver were studied under conditions of antioxidant insufficiency (AOI) which was modelled by exclusion of alpha-tocopherol from the animals' ration. An insignificant accumulation of microsomal diene conjugates and schiff bases against a sharp increase of the ability to the prooxidant stimulated LPO in vitro took place. A significant decrease of membrane lipid microviscosity and a change in surface properties of microsomal membranes of rats with AOI was determined. Absence of alpha-tocopherol in the ration was accompanied by a significant change in the content of separate isoforms of cytochrome P-450 exhibited in growth of a polypeptide with m. w. 54 kDa and the lowering of proteins with m. w. 48 and 50 kDa. Less intensive quenching of tryptophan fluorescence by acrylamide was also revealed, which testified to a lower accessibility of the quencher to membrane proteins or their fluorophore sites. Modification of lipid composition and of physicochemical properties of the rat liver membrane microsomes which was observed at AOI was significantly correlated by pretreatment with the antioxidant 4-methyl-2,6-ditretbutylphenol (ionol).
-
Demonstration of conjugated dopamine in monkey CSF by gas chromatography-mass spectrometry
Energy Technology Data Exchange (ETDEWEB)
Elchisak, M A; Powers, K H; Ebert, M H
1982-09-01
A method for measuring unconjugated and conjugated dopamine in body tissues and fluids is described. Conjugated dopamine was hydrolyzed in acid to unconjugated dopamine, separated from the sample matrix by alumina chromatography, and assayed by gas chromatography-mass spectrometry. Conjugated dopamine was detected in greater concentrations than unconjugated dopamine in CSF taken from lateral ventricle or thecal sac of the Rhesus monkey. Haloperidol administration did not increase the levels of conjugated dopamine in lumbar CSF.
-
Social behaviour and decision making in bacterial conjugation
Directory of Open Access Journals (Sweden)
Günther eKoraimann
2014-04-01
Full Text Available Bacteria frequently acquire novel genes by HGT (horizontal gene transfer. HGT through the process of bacterial conjugation is highly efficient and depends on the presence of conjugative plasmids (CPs or integrated conjugative elements (ICEs that provide the necessary genes for DNA transmission. This review focuses on recent advancements in our understanding of ssDNA transfer systems and regulatory networks ensuring timely and spatially controlled DNA transfer (tra gene expression. As will become obvious by comparing different systems, by default, tra genes are shut off in cells in which conjugative elements are present. Only when conditions are optimal, donor cells – through epigenetic alleviation of negatively acting roadblocks and direct stimulation of DNA transfer genes – become transfer competent. These transfer competent cells have developmentally transformed into specialized cells capable of secreting ssDNA via a T4S (type IV secretion complex directly into recipient cells. Intriguingly, even under optimal conditions, only a fraction of the population undergoes this transition, a finding that indicates specialization and cooperative, social behavior. Thereby, at the population level, the metabolic burden and other negative consequences of tra gene expression are greatly reduced without compromising the ability to horizontally transfer genes to novel bacterial hosts. This undoubtedly intelligent strategy may explain why conjugative elements – CPs and ICEs – have been successfully kept in and evolved with bacteria to constitute a major driving force of bacterial evolution.
-
Factors contributing to the immunogenicity of meningococcal conjugate vaccines
Bröker, Michael; Berti, Francesco; Costantino, Paolo
2016-01-01
ABSTRACT Various glycoprotein conjugate vaccines have been developed for the prevention of invasive meningococcal disease, having significant advantages over pure polysaccharide vaccines. One of the most important features of the conjugate vaccines is the induction of a T-cell dependent immune response, which enables both the induction of immune memory and a booster response after repeated immunization. The nature of the carrier protein to which the polysaccharides are chemically linked, is often regarded as the main component of the vaccine in determining its immunogenicity. However, other factors can have a significant impact on the vaccine's profile. In this review, we explore the physico-chemical properties of meningococcal conjugate vaccines, which can significantly contribute to the vaccine's immunogenicity. We demonstrate that the carrier is not the sole determining factor of the vaccine's profile, but, moreover, that the conjugate vaccine's immunogenicity is the result of multiple physico-chemical structures and characteristics. PMID:26934310
-
Directory of Open Access Journals (Sweden)
Jiali Xing
Full Text Available Antioxidant activity of lactic acid bacteria is associated with multiple health-protective effects. Traditional indexes of chemical antioxidant activities poorly reflect the antioxidant effects of these bacteria in vivo. Cellular antioxidant activity (CAA assay was used in this study to determine the antioxidant activity of cell-free supernatants (CFSs of 10 Lactobacillus strains. The performance of the CAA assay was compared with that of four chemical antioxidant activity assays, namely, DPPH radical scavenging, hydroxyl radical scavenging (HRS, reducing power (RP, and inhibition of linoleic acid peroxidation (ILAP. Results of the CAA assay were associated with those of DPPH and ILAP assays, but not with those of RP and HRS assays. The inter- and intra-specific antioxidant activities of CFS were characterized by chemical and CAA assays. L. rhamnosus CCFM 1107 displayed a high antioxidative effect similar to positive control L. rhamnosus GG ATCC 53103 in all of the assays. The CAA assay is a potential method for the detection of antioxidant activities of lactobacilli CFSs.
-
Wang, Ying; Yang, Meng; Lee, Sang-Gil; Davis, Catherine G; Kenny, Anne; Koo, Sung I; Chun, Ock K
2012-12-01
Increased plasma total antioxidant capacity (TAC) has been associated with a high consumption of fruits and vegetables. However, limited information is available on whether plasma TAC reflects the dietary intake of antioxidants and the levels of individual antioxidants in plasma. By using three different assays, the study aimed to determine if plasma TAC can effectively predict dietary intake of antioxidants and plasma antioxidant status. Forty overweight and apparently healthy postmenopausal women were recruited. Seven-day food records and 12-h fasting blood samples were collected for dietary and plasma antioxidant assessments. Plasma TAC was determined by vitamin C equivalent antioxidant capacity (VCEAC), ferric-reducing ability of plasma (FRAP) and oxygen radical absorbance capacity (ORAC) assays. TAC values determined by VCEAC were highly correlated with FRAP (r=0.79, Pantioxidants and represents more closely the plasma antioxidant levels than ORAC and FRAP. Copyright © 2012 Elsevier Inc. All rights reserved.
-
Efficient conjugate gradient algorithms for computation of the manipulator forward dynamics
Fijany, Amir; Scheid, Robert E.
1989-01-01
The applicability of conjugate gradient algorithms for computation of the manipulator forward dynamics is investigated. The redundancies in the previously proposed conjugate gradient algorithm are analyzed. A new version is developed which, by avoiding these redundancies, achieves a significantly greater efficiency. A preconditioned conjugate gradient algorithm is also presented. A diagonal matrix whose elements are the diagonal elements of the inertia matrix is proposed as the preconditioner. In order to increase the computational efficiency, an algorithm is developed which exploits the synergism between the computation of the diagonal elements of the inertia matrix and that required by the conjugate gradient algorithm.
-
Functional Hybrid Biomaterials based on Peptide-Polymer Conjugates for Nanomedicine
Shu, Jessica Yo
The focus of this dissertation is the design, synthesis and characterization of hybrid functional biomaterials based on peptide-polymer conjugates for nanomedicine. Generating synthetic materials with properties comparable to or superior than those found in nature has been a "holy grail" for the materials community. Man-made materials are still rather simplistic when compared to the chemical and structural complexity of a cell. Peptide-polymer conjugates have the potential to combine the advantages of the biological and synthetic worlds---that is they can combine the precise chemical structure and diverse functionality of biomolecules with the stability and processibility of synthetic polymers. As a new family of soft matter, they may lead to materials with novel properties that have yet to be realized with either of the components alone. In order for peptide-polymer conjugates to reach their full potential as useful materials, the structure and function of the peptide should be maintained upon polymer conjugation. The success in achieving desirable, functional assemblies relies on fundamentally understanding the interactions between each building block and delicately balancing and manipulating these interactions to achieve targeted assemblies without interfering with designed structures and functionalities. Such fundamental studies of peptide-polymer interactions were investigated as the nature of the polymer (hydrophilic vs. hydrophobic) and the site of its conjugation (end-conjugation vs. side-conjugation) were varied. The fundamental knowledge gained was then applied to the design of amphiphiles that self-assemble to form stable functional micelles. The micelles exhibited exceptional monodispersity and long-term stability, which is atypical of self-assembled systems. Thus such micelles based on amphiphilic peptide-polymer conjugates may meet many current demands in nanomedicine, in particular for drug delivery of hydrophobic anti-cancer therapeutics. Lastly
-
Preparation and characterization of microspheres of albumin-heparin conjugates
Kwon, Glen S.; Bae, You Han; Kim, Sung Wan; Cremers, Harry; Cremers, H.F.M.; Feijen, Jan
1991-01-01
Albumin-heparin microspheres have been prepared as a new drug carrier. A soluble albumin-heparin conjugate was synthesized by forming amide bonds between human serum albumin and heparin. After purification the albumin-heparin conjugate was crosslinked in a water-in-oil emulsion to form
-
Phase-conjugate optical coherence tomography
International Nuclear Information System (INIS)
Erkmen, Baris I.; Shapiro, Jeffrey H.
2006-01-01
Quantum optical coherence tomography (Q-OCT) offers a factor-of-2 improvement in axial resolution and the advantage of even-order dispersion cancellation when it is compared to conventional OCT (C-OCT). These features have been ascribed to the nonclassical nature of the biphoton state employed in the former, as opposed to the classical state used in the latter. Phase-conjugate OCT (PC-OCT) shows that nonclassical light is not necessary to reap Q-OCT's advantages. PC-OCT uses classical-state signal and reference beams, which have a phase-sensitive cross correlation, together with phase conjugation to achieve the axial resolution and even-order dispersion cancellation of Q-OCT with a signal-to-noise ratio that can be comparable to that of C-OCT
-
Modern methods for the synthesis of peptide-oligonucleotide conjugates
International Nuclear Information System (INIS)
Zubin, Evgenii M; Oretskaya, Tat'yana S; Romanova, Elena A
2002-01-01
The published data on the methods of chemical solution and solid-phase synthesis of peptide-oligonucleotide conjugates are reviewed. The known methods are systematised and their advantages and disadvantages are considered. The approaches to the solution synthesis of peptide-oligonucleotide conjugates are systematised according to the type of chemical bonds between the fragments, whereas those to the solid-phase synthesis are classified according to the procedure used for the preparation of conjugates, viz., stepwise elongation of oligonucleotide and peptide chains on the same polymeric support or solid-phase condensation of two presynthesised fragments. The bibliography includes 141 references.
-
Perfect lensing with phase-conjugating surfaces: toward practical realization
International Nuclear Information System (INIS)
Maslovski, Stanislav; Tretyakov, Sergei
2012-01-01
It is theoretically known that a pair of phase-conjugating surfaces can function as a perfect lens, focusing propagating waves and enhancing evanescent waves. However, the known experimental approaches based on thin sheets of nonlinear materials cannot fully realize the required phase conjugation boundary condition. In this paper, we show that the ideal phase-conjugating surface is, in principle, physically realizable and investigate the necessary properties of nonlinear and nonreciprocal particles which can be used to build a perfect lens system. The physical principle of the lens operation is discussed in detail and directions of possible experimental realizations are outlined. (paper)
-
[Role of proton-motive force in the conjugative DNA transport in Staphylococci].
Gavriliuk, V G; Vinnikov, A I
1997-01-01
Sensitivity of the conjugative process in staphylococci to the action of uncouplers of oxidative phosphorylation and inhibitors of electron transport systems have been proved, that testifies to the energy-dependent character of conjugative transport of DNA. Proceeding of the conjugation process depends upon the generation of delta microH+ on the membrane of both the donor and recipient cells. contribution of protonmotive forces to providing for the transfer of plasmids during conjugation to staphylococci has been defined.
-
PREPARATION OF CHEMICALLY WELL-DEFINED CARBOHYDRATE DENDRIMER CONJUGATES
DEFF Research Database (Denmark)
2004-01-01
A method for the synthesis of dendrimer conjugates having a well-defined chemical structure, comprising one or more carbohydrate moieties and one or more immunomodulating substances coupled to a dendrimer, is presented. First, the carbohydrate is bound to the dendrimer in a chemoselective manner...... conjugates and their use in vaccination, production of antibodies, high throughput screening, diagnostic assays and libraries....
-
Thermal Stability of siRNA Modulates Aptamer- conjugated siRNA Inhibition
Directory of Open Access Journals (Sweden)
Alexey Berezhnoy
2012-01-01
Full Text Available Oligonucleotide aptamer-mediated in vivo cell targeting of small interfering RNAs (siRNAs is emerging as a useful approach to enhance the efficacy and reduce the adverse effects resulting from siRNA-mediated genetic interference. A current main impediment in aptamer-mediated siRNA targeting is that the activity of the siRNA is often compromised when conjugated to an aptamer, often requiring labor intensive and time consuming design and testing of multiple configurations to identify a conjugate in which the siRNA activity has not been significantly reduced. Here, we show that the thermal stability of the siRNA is an important parameter of siRNA activity in its conjugated form, and that siRNAs with lower melting temperature (Tm are not or are minimally affected when conjugated to the 3′ end of 2′F-pyrimidine-modified aptamers. In addition, the configuration of the aptamer-siRNA conjugate retains activity comparable with the free siRNA duplex when the passenger strand is co-transcribed with the aptamer and 3′ overhangs on the passenger strand are removed. The approach described in this paper significantly reduces the time and effort necessary to screening siRNA sequences that retain biological activity upon aptamer conjugation, facilitating the process of identifying candidate aptamer-siRNA conjugates suitable for in vivo testing.
-
Dual peptide conjugation strategy for improved cellular uptake and mitochondria targeting.
Lin, Ran; Zhang, Pengcheng; Cheetham, Andrew G; Walston, Jeremy; Abadir, Peter; Cui, Honggang
2015-01-21
Mitochondria are critical regulators of cellular function and survival. Delivery of therapeutic and diagnostic agents into mitochondria is a challenging task in modern pharmacology because the molecule to be delivered needs to first overcome the cell membrane barrier and then be able to actively target the intracellular organelle. Current strategy of conjugating either a cell penetrating peptide (CPP) or a subcellular targeting sequence to the molecule of interest only has limited success. We report here a dual peptide conjugation strategy to achieve effective delivery of a non-membrane-penetrating dye 5-carboxyfluorescein (5-FAM) into mitochondria through the incorporation of both a mitochondrial targeting sequence (MTS) and a CPP into one conjugated molecule. Notably, circular dichroism studies reveal that the combined use of α-helix and PPII-like secondary structures has an unexpected, synergistic contribution to the internalization of the conjugate. Our results suggest that although the use of positively charged MTS peptide allows for improved targeting of mitochondria, with MTS alone it showed poor cellular uptake. With further covalent linkage of the MTS-5-FAM conjugate to a CPP sequence (R8), the dually conjugated molecule was found to show both improved cellular uptake and effective mitochondria targeting. We believe these results offer important insight into the rational design of peptide conjugates for intracellular delivery.
-
Peptide-conjugated micelles as a targeting nanocarrier for gene delivery
Energy Technology Data Exchange (ETDEWEB)
Lin, Wen Jen, E-mail: wjlin@ntu.edu.tw; Chien, Wei Hsuan [National Taiwan University, School of Pharmacy, Graduate Institute of Pharmaceutical Sciences (China)
2015-09-15
The aim of this study was to develop peptide-conjugated micelles possessing epidermal growth factor receptor (EGFR) targeting ability for gene delivery. A sequence-modified dodecylpeptide, GE11(2R), with enhancing EGF receptor binding affinity, was applied in this study as a targeting ligand. The active targeting micelles were composed of poly(d,l-lactide-co-glycolide)-poly(ethylene glycol) (PLGA-PEG) copolymer conjugated with GE11(2R)-peptide. The particle sizes of peptide-free and peptide-conjugated micelles were 277.0 ± 5.1 and 308.7 ± 14.5 nm, respectively. The peptide-conjugated micelles demonstrated the cellular uptake significantly higher than peptide-free micelles in EGFR high-expressed MDA-MB-231 and MDA-MB-468 cells due to GE11(2R)-peptide specificity. Furthermore, the peptide-conjugated micelles were able to encapsulate plasmid DNA and expressed cellular transfection higher than peptide-free micelles in EGFR high-expressed cells. The EGFR-targeting delivery micelles enhanced DNA internalized into cells and achieved higher cellular transfection in EGFR high-expressed cells.
-
Nanostructured Conjugated Polymers for Energy-Related Applications beyond Solar Cells.
Xie, Jian; Zhao, Cui-E; Lin, Zong-Qiong; Gu, Pei-Yang; Zhang, Qichun
2016-05-20
To meet the ever-increasing requirements for the next generation of sustainable and versatile energy-related devices, conjugated polymers, which have potential advantages over small molecules and inorganic materials, are among the most promising types of green candidates. The properties of conjugated polymers can be tuned through modification of the structure and incorporation of different functional moieties. In addition, superior performances can be achieved as a result of the advantages of nanostructures, such as their large surface areas and the shortened pathways for charge transfer. Therefore, nanostructured conjugated polymers with different properties can be obtained to be applied in different energy-related organic devices. This review focuses on the application and performance of the recently reported nanostructured conjugated polymers for high-performance devices, including rechargeable lithium batteries, microbial fuel cells (MFCs), thermoelectric generators, and photocatalytic systems. The design strategies, reaction mechanisms, advantages, and limitations of nanostructured conjugated polymers are further discussed in each section. Finally, possible routes to improve the performances of the current systems are also included in the conclusion. © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
-
Women experiencing the intergenerationality of conjugal violence
Directory of Open Access Journals (Sweden)
Gilvânia Patrícia do Nascimento Paixão
2015-10-01
Full Text Available Objective: to analyze the family relationship, in childhood and adolescence, of women who experience conjugal violence.Method: qualitative study. Interviews were held with 19 women, who were experiencing conjugal violence, and who were resident in a community in Salvador, Bahia, Brazil. The project was approved by the Research Ethics Committee (N. 42/2011.Results: the data was organized using the Discourse of the Collective Subject, identifying the summary central ideas: they witnessed violence between their parents; they suffered repercussions from the violence between their parents: they were angry about the mother's submission to her partner; and they reproduced the conjugal violence. The discourse showed that the women witnessed, in childhood and adolescence, violence between their parents, and were injured both physically and psychologically. As a result of the mother's submission, feelings of anger arose in the children. However, in the adult phase of their own lives, they noticed that their conjugal life resembled that of their parents, reproducing the violence.Conclusion: investment is necessary in strategies designed to break inter-generational violence, and the health professionals are important in this process, as it is a phenomenon with repercussions in health. Because they work in the Family Health Strategy, which focuses on the prevention of harm and illness, health promotion and interdepartmentality, the nurses are essential in the process of preventing and confronting this phenomenon.
-
A nanodiamond-fluorescein conjugate for cell studies
Pedroso-Santana, Seidy; Fleitas-Salazar, Noralvis; Sarabia-Sainz, Andrei; Silva-Campa, Erika; Burgara-Estrella, Alexel; Angulo-Molina, Aracely; Melendrez, Rodrigo; Pedroza-Montero, Martin; Riera, Raul
2018-03-01
The use of nanodiamonds in studies with living systems generally involves the modification of their surfaces with functional groups. Fluorescent molecules can be attached to these groups, so that one can know the exact position of the particles in each moment of the interaction with the cells. Here we modify the surface of detonation nanodiamonds and nitrogen-vacancy center nanodiamonds using carboxylation and hydroxylation procedures. Subsequent reactions with silicates and cysteine, before addition of fluorescein allow to obtain fluorescent nano-conjugates. We used confocal microscopy to observe the position of nanodiamonds interacting with HeLa cells. At 3 h post-incubation the green fluorescence is localized in extracellular rounded like-vesicles assemblies while at 24 h the conjugates can be observed inside the cells. The measurement of the fluorescence emitted by both conjugates allowed to find an enhanced emission of fluorescein isothiocyanate (FITC) when the nitrogen-vacancy center is present. We propose the existence of a fluorescence enhancement by electron transference process. The procedure described in this work allows the functionalization of nanodiamonds with FITC and other molecules using functional surface groups and small size mediators. Also, as was proved in our work, the nanodiamond-fluorescein conjugates can be used to track nanoparticles position within the cell. Localization studies are particularly important for drug delivery applications of nanodiamonds.
-
Antioxidant Properties of Probiotic Bacteria
Directory of Open Access Journals (Sweden)
Yang Wang
2017-05-01
Full Text Available Oxidative stress defines a condition in which the prooxidant–antioxidant balance in the cell is disturbed, resulting in DNA hydroxylation, protein denaturation, lipid peroxidation, and apoptosis, ultimately compromising cells’ viability. Probiotics have been known for many beneficial health effects, and the consumption of probiotics alone or in food shows that strain-specific probiotics can present antioxidant activity and reduce damages caused by oxidation. However, the oxidation-resistant ability of probiotics, especially the underling mechanisms, is not properly understood. In this view, there is interest to figure out the antioxidant property of probiotics and summarize the mode of action of probiotic bacteria in antioxidation. Therefore, in the present paper, the antioxidant mechanisms of probiotics have been reviewed in terms of their ability to improve the antioxidant system and their ability to decrease radical generation. Since in recent years, oxidative stress has been associated with an altered gut microbiota, the effects of probiotics on intestinal flora composition are also elaborated.
-
Antioxidant Properties of Probiotic Bacteria.
Wang, Yang; Wu, Yanping; Wang, Yuanyuan; Xu, Han; Mei, Xiaoqiang; Yu, Dongyou; Wang, Yibing; Li, Weifen
2017-05-19
Oxidative stress defines a condition in which the prooxidant-antioxidant balance in the cell is disturbed, resulting in DNA hydroxylation, protein denaturation, lipid peroxidation, and apoptosis, ultimately compromising cells' viability. Probiotics have been known for many beneficial health effects, and the consumption of probiotics alone or in food shows that strain-specific probiotics can present antioxidant activity and reduce damages caused by oxidation. However, the oxidation-resistant ability of probiotics, especially the underling mechanisms, is not properly understood. In this view, there is interest to figure out the antioxidant property of probiotics and summarize the mode of action of probiotic bacteria in antioxidation. Therefore, in the present paper, the antioxidant mechanisms of probiotics have been reviewed in terms of their ability to improve the antioxidant system and their ability to decrease radical generation. Since in recent years, oxidative stress has been associated with an altered gut microbiota, the effects of probiotics on intestinal flora composition are also elaborated.
-
21 CFR 862.1115 - Urinary bilirubin and its conjugates (nonquantitative) test system.
2010-04-01
... 21 Food and Drugs 8 2010-04-01 2010-04-01 false Urinary bilirubin and its conjugates... Clinical Chemistry Test Systems § 862.1115 Urinary bilirubin and its conjugates (nonquantitative) test system. (a) Identification. A urinary bilirubin and its conjugates (nonquantitative) test system is a...
-
Directory of Open Access Journals (Sweden)
Mykhailo Reheda
2018-04-01
Full Text Available We have analyzed the results of the research of changes in indices of lipid peroxidation (conjugated dienes and malondialdehide and antioxidant (superoxide dismutase, catalase, ceruloplasmin systems in guinea pigs’ periodontal soft tissues in experimental bronchial asthma in the dynamics of asthma under conditions of chronic periodontitis and correction of these changes with thiotriazoline. The research was conducted on 50 male guinea pigs weighting 250-270 g, divided into 5 groups: I – intact guinea pigs (n=10, II – guinea pigs (n=10 with asthma under conditions of chronic periodontitis before correction (4th day, III - guinea pigs (n=10 with asthma under conditions of chronic periodontitis before correction (18th day, IV - guinea pigs (n=10 with asthma under conditions of chronic periodontitis before correction (25 th day, V - guinea pigs (n=10 with asthma under conditions of chronic periodontitis after correction (25th day. The results of experimental studies showed the significant increase of conjugated dienes and malondialdehide levels in animal’s periodontal soft tissues at all observed stages of asthma development under conditions of chronic periodontitis before the correction as compared with control group. Intensive synthesis of LPO’s products caused increase on 4th day with further decrease on 18th and 25th days of activity levels of superoxide dismutase, catalase and ceruloplasmin in animal’s periodontal soft tissues before the correction as compared with indices of the control group. After correction with thiotriazoline the the results showed decrease of indices of LPO and increase of activity levels of antioxidant enzymes.
-
Energy Technology Data Exchange (ETDEWEB)
Kim, Eun-Kyung; Shrestha, Nabeen K. [Department of Chemistry, Hanyang University, Seoul 133-791 (Korea, Republic of); Lee, Wonjoo [Department of Chemistry, Hanyang University, Seoul 133-791 (Korea, Republic of); Department of Defense Ammunitions, Daeduk College, Daejeon 305-715 (Korea, Republic of); Cai, Gangri, E-mail: caigangri@naver.com [Department of Applied Chemistry, TianJin University of Technology, Tianjin 300384 (China); Department of Chemistry, Hanyang University, Seoul 133-791 (Korea, Republic of); Han, Sung-Hwan, E-mail: shhan@hanyang.ac.kr [Department of Chemistry, Hanyang University, Seoul 133-791 (Korea, Republic of)
2015-04-01
Manganese dioxide (MnO{sub 2}) thin films are deposited electrochemically on an indium–tin-oxide (ITO) electrode using aqueous bath in presence of conjugated water soluble sulfonated polyaniline (SPAN) or a non-conjugated polyacrylic acid (PAA) polyelectrolyte surfactant. The surface morphology and nature of the electrodeposited MnO{sub 2} films are found to be influenced strongly by the amount and type of polyelectrolyte in the deposition bath. Increasing the SPAN concentration, a porous structure resulting from the reduction of voids between the MnO{sub 2} nano-flakes is obtained. In contrast, by increasing the PAA concentration, dense and spherical MnO{sub 2} nanostructures have been deposited. These results may be caused by initiation of different kinetics and orientation of nucleation of MnO{sub 2} deposits on ITO surface in presence of different types of polyelectrolytes. Cyclic voltammetry study of these films shows the supercapacitor behavior. The porous MnO{sub 2} films grown from the SPAN containing electrolyte demonstrates a specific capacitance of 368.53 F/g at scan rate of 10 mV/s, which is approximately 10 times higher (i.e., 30.29 F/g) than that of the spherical MnO{sub 2} dense films grown from PAA containing electrolyte. - Highlights: • Conjugated/non-conjugated polyelectrolytes were used in deposition of MnO{sub 2}. • The two kinds of MnO{sub 2} film showed entirely different morphology. • Conjugated polyelectrolyte worked as template and also affected the growth rate. • Non-conducting polyelectrolyte could work as template but hindered MnO{sub 2} growth. • The specific capacitance of MnO{sub 2}–S was 10 times higher than MnO{sub 2}–P.
-
Beekwilder, M.J.; Jonker, H.H.; Hall, R.D.; Meer, van der I.M.; Vos, de C.H.
2005-01-01
The presence of antioxidant compounds can be considered as a quality parameter for edible fruit. In this paper, we studied the antioxidant compounds in raspberry (Rubus idaeus) fruits by high-performance liquid chromatography (HPLC) coupled to an on-line postcolumn antioxidant detection system. Both
-
Peptide- and saccharide-conjugated dendrimers for targeted drug delivery: a concise review
Liu, Jie; Gray, Warren D.; Davis, Michael E.; Luo, Ying
2012-01-01
Dendrimers comprise a category of branched materials with diverse functions that can be constructed with defined architectural and chemical structures. When decorated with bioactive ligands made of peptides and saccharides through peripheral chemical groups, dendrimer conjugates are turned into nanomaterials possessing attractive binding properties with the cognate receptors. At the cellular level, bioactive dendrimer conjugates can interact with cells with avidity and selectivity, and this function has particularly stimulated interests in investigating the targeting potential of dendrimer materials for the design of drug delivery systems. In addition, bioactive dendrimer conjugates have so far been studied for their versatile capabilities to enhance stability, solubility and absorption of various types of therapeutics. This review presents a brief discussion on three aspects of the recent studies to use peptide- and saccharide-conjugated dendrimers for drug delivery: (i) synthesis methods, (ii) cell- and tissue-targeting properties and (iii) applications of conjugated dendrimers in drug delivery nanodevices. With more studies to elucidate the structure–function relationship of ligand–dendrimer conjugates in transporting drugs, the conjugated dendrimers hold promise to facilitate targeted delivery and improve drug efficacy for discovery and development of modern pharmaceutics. PMID:23741608
-
Rapid modification of retroviruses using lipid conjugates
International Nuclear Information System (INIS)
Mukherjee, Nimisha G; Le Doux, Joseph M; Andrew Lyon, L
2009-01-01
Methods are needed to manipulate natural nanoparticles. Viruses are particularly interesting because they can act as therapeutic cellular delivery agents. Here we examine a new method for rapidly modifying retroviruses that uses lipid conjugates composed of a lipid anchor (1,2-distearoyl-sn-glycero-3-phosphoethanolamine), a polyethylene glycol chain, and biotin. The conjugates rapidly and stably modified retroviruses and enabled them to bind streptavidin. The implication of this work for modifying viruses for gene therapy and vaccination protocols is discussed.
-
CONJUGATED POLYMERS AND POLYELECTROLYTES IN SOLAR PHOTOCONVERSION, Final Technical Report
Energy Technology Data Exchange (ETDEWEB)
Schanze, Kirk S [University of Florida
2014-08-05
This DOE-supported program investigated the fundamental properties of conjugated polyelectrolytes, with emphasis placed on studies of excited state energy transport, self-assembly into conjugated polyelectroyte (CPE) based films and colloids, and exciton transport and charge injection in CPE films constructed atop wide bandgap semiconductors. In the most recent grant period we have also extended efforts to examine the properties of low-bandgap donor-acceptor conjugated polyelectrolytes that feature strong visible light absorption and the ability to adsorb to metal-oxide interfaces.
-
Antioxidant activity of lichen Cetraria aculeata
Directory of Open Access Journals (Sweden)
Tomović Jovica
2016-01-01
Full Text Available The aim of the present study is to investigate the antioxidant properties of the lichen Cetraria aculeata. Antioxidant activity of the methanol and ethyl acetate extracts of lichen was tested by different methods including determination of total phenolics content, determination of total antioxidant capacity, DPPH free radical scavenging activity, inhibitory activity towards lipid peroxidation, ferrous ion chelating ability and hydroxyl radical scavenging activity. The extracts of the lichen C. aculeata showed significant antioxidant activity. The methanol extract showed higher values for total phenolics and total antioxidant capacity compared to the ethyl acetate extract, while the ethyl acetate extract demonstrated better results for DPPH radical scavenging, inhibitory activity towards lipid peroxidation, chelating ability and hydroxyl radical scavenging than the methanol extract. This is the first report of the antioxidant properties of Cetraria aculeata growing in Serbia. The results of antioxidant activity indicate the application of this lichen as source of natural antioxidants that could be used as a possible food supplement, in the pharmaceutical industry and in the treatment of various diseases.
-
Synthesis and Characterization of Water-soluble Conjugates of Cabazitaxel Hemiesters-Dextran.
Parhizkar, Elahehnaz; Ahmadi, Fatemeh; Daneshamouz, Saeid; Mohammadi-Samani, Soliman; Sakhteman, Amirhossein; Parhizkar, Golnaz
2017-11-24
Cabazitaxel (CTX) is a second- generation taxane derivative, a class of potent anticancer drugs with very low water solubility. CTX is used in patients with resistant prostate cancer unresponsive to the first generation taxane, docetaxel. Currently marketed formulations of CTX contain high concentrations of surfactant and ethanol, which cause severe hypersensitivity reactions in patients. In order to increase its solubility, two hemiester analogs; CTX-succinate and CTX-glutarate were synthesized and characterized. To improve the solubility of hemiesters even more, dextran as a biocompatible polymer was also conjugated to hemiester analogs. MTT assay was performed on MCF-7 cell line to evaluate the cytotoxicity effect of hemiesters and conjugates. Based on the results, hemiester analogs increased water solubility of the drug up to about 3 and 8 fold. Conjugation to dextran enhanced the CTX solubility to more than 1500 fold. These conjugates released the conjugated CTX in less than 24 hours in a pH dependent manner and showed proper hemocompatibility characteristics. The hemiesters had approximately similar cytotoxicity in comparison with CTX and the dextran conjugates showed higher cytotoxicity effect on MCF-7 cell line. Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
-
Hemoglobin Detection on a Microfluidic Sensor Chip with a Partially Conjugated Polymer
International Nuclear Information System (INIS)
Eo, Soo Han; Won, Kwang Jae; Song, Simon; Yoon, Bora; Kim, Jong Man
2010-01-01
The development of efficient chemosensors based on the conjugated polymers has been the central focus of a large number of recent research programs. The presence of extensively delocalized electrons and conformational restrictions of the backbone structures make conjugated polymers attractive sensory materials. In these polymers, molecular recognition events influence electronic absorption and emission properties. Thus, a wide variety of conjugated polymer-based sensors have been investigated. However, the majority of the conjugated polymer sensors described to date have been explored in the form of solutions or thin films. Most biologically interesting target molecules, such as proteins, carbohydrates, nucleic acids, or ions, are only soluble in water. Thus, it is desirable to use water-soluble conjugated polymers as sensor matrices. In general, in order to make water-soluble conjugated polymers tedious procedures are required since most synthetic methods developed for this purpose are incompatible with sidechain functionalities. Accordingly, protecting group strategies are required to prepare polymers with requisite functional groups that foster water solubility
-
Diclofenac in Arabidopsis cells: Rapid formation of conjugates.
Fu, Qiuguo; Ye, Qingfu; Zhang, Jianbo; Richards, Jaben; Borchardt, Dan; Gan, Jay
2017-03-01
Pharmaceutical and personal care products (PPCPs) are continuously introduced into the soil-plant system, through practices such as agronomic use of reclaimed water and biosolids containing these trace contaminants. Plants may accumulate PPCPs from soil, serving as a conduit for human exposure. Metabolism likely controls the final accumulation of PPCPs in plants, but is in general poorly understood for emerging contaminants. In this study, we used diclofenac as a model compound, and employed 14 C tracing, and time-of-flight (TOF) and triple quadruple (QqQ) mass spectrometers to unravel its metabolism pathways in Arabidopsis thaliana cells. We further validated the primary metabolites in Arabidopsis seedlings. Diclofenac was quickly taken up into A. thaliana cells. Phase I metabolism involved hydroxylation and successive oxidation and cyclization reactions. However, Phase I metabolites did not accumulate appreciably; they were instead rapidly conjugated with sulfate, glucose, and glutamic acid through Phase II metabolism. In particular, diclofenac parent was directly conjugated with glutamic acid, with acyl-glutamatyl-diclofenac accounting for >70% of the extractable metabolites after 120-h incubation. In addition, at the end of incubation, >40% of the spiked diclofenac was in the non-extractable form, suggesting extensive sequestration into cell matter. The rapid formation of non-extractable residue and dominance of diclofenac-glutamate conjugate uncover previously unknown metabolism pathways for diclofenac. In particular, the rapid conjugation of parent highlights the need to consider conjugates of emerging contaminants in higher plants, and their biological activity and human health implications. Copyright © 2016 Elsevier Ltd. All rights reserved.
-
Conjugate Meningococcal Vaccines Development: GSK Biologicals Experience
Directory of Open Access Journals (Sweden)
Jacqueline M. Miller
2011-01-01
Full Text Available Meningococcal diseases are serious threats to global health, and new vaccines specifically tailored to meet the age-related needs of various geographical areas are required. This paper focuses on the meningococcal conjugate vaccines developed by GSK Biologicals. Two combined conjugate vaccines were developed to help protect infants and young children in countries where the incidence of meningococcal serogroup C or serogroup C and Y disease is important: Hib-MenC-TT vaccine, which offers protection against Haemophilus influenzae type b and Neisseria meningitidis serogroup C diseases, is approved in several countries; and Hib-MenCY-TT vaccine, which adds N. meningitidis serogroup Y antigen, is currently in the final stages of development. Additionally, a tetravalent conjugate vaccine (MenACWY-TT designed to help protect against four meningococcal serogroups is presently being evaluated for global use in all age groups. All of these vaccines were shown to be highly immunogenic and to have clinically acceptable safety profiles.
-
Conjugate Meningococcal Vaccines Development: GSK Biologicals Experience
Miller, Jacqueline M.; Mesaros, Narcisa; Van Der Wielen, Marie; Baine, Yaela
2011-01-01
Meningococcal diseases are serious threats to global health, and new vaccines specifically tailored to meet the age-related needs of various geographical areas are required. This paper focuses on the meningococcal conjugate vaccines developed by GSK Biologicals. Two combined conjugate vaccines were developed to help protect infants and young children in countries where the incidence of meningococcal serogroup C or serogroup C and Y disease is important: Hib-MenC-TT vaccine, which offers protection against Haemophilus influenzae type b and Neisseria meningitidis serogroup C diseases, is approved in several countries; and Hib-MenCY-TT vaccine, which adds N. meningitidis serogroup Y antigen, is currently in the final stages of development. Additionally, a tetravalent conjugate vaccine (MenACWY-TT) designed to help protect against four meningococcal serogroups is presently being evaluated for global use in all age groups. All of these vaccines were shown to be highly immunogenic and to have clinically acceptable safety profiles. PMID:21991444
-
Degradable conjugated polymers for the selective sorting of semiconducting carbon nanotubes
Gopalan, Padma; Arnold, Michael Scott; Kansiusarulsamy, Catherine Kanimozhi; Brady, Gerald Joseph; Shea, Matthew John
2018-04-10
Conjugated polymers composed of bi-pyridine units linked to 9,9-dialkyl fluorenyl-2,7-diyl units via imine linkages along the polymer backbone are provided. Also provided are semiconducting single-walled carbon nanotubes coated with the conjugated polymers and methods of sorting and separating s-SWCNTs from a sample comprising a mixture of s-SWCNTs and metallic single-walled carbon nanotubes using the conjugated polymers.
-
Firth, David; Bell, Leonard; Squires, Martin; Estdale, Sian; McKee, Colin
2015-09-15
We present the demonstration of a rapid "middle-up" liquid chromatography mass spectrometry (LC-MS)-based workflow for use in the characterization of thiol-conjugated maleimidocaproyl-monomethyl auristatin F (mcMMAF) and valine-citrulline-monomethyl auristatin E (vcMMAE) antibody-drug conjugates. Deconvoluted spectra were generated following a combination of deglycosylation, IdeS (immunoglobulin-degrading enzyme from Streptococcus pyogenes) digestion, and reduction steps that provide a visual representation of the product for rapid lot-to-lot comparison-a means to quickly assess the integrity of the antibody structure and the applied conjugation chemistry by mass. The relative abundance of the detected ions also offer information regarding differences in drug conjugation levels between samples, and the average drug-antibody ratio can be calculated. The approach requires little material (<100 μg) and, thus, is amenable to small-scale process development testing or as an early component of a complete characterization project facilitating informed decision making regarding which aspects of a molecule might need to be examined in more detail by orthogonal methodologies. Copyright © 2015 Elsevier Inc. All rights reserved.
-
Non-Andersonian conjugate strike-slip faults: Observations, theory, and tectonic implications
International Nuclear Information System (INIS)
Yin, A; Taylor, M H
2008-01-01
Formation of conjugate strike-slip faults is commonly explained by the Anderson fault theory, which predicts a X-shaped conjugate fault pattern with an intersection angle of ∼30 degrees between the maximum compressive stress and the faults. However, major conjugate faults in Cenozoic collisional orogens, such as the eastern Alps, western Mongolia, eastern Turkey, northern Iran, northeastern Afghanistan, and central Tibet, contradict the theory in that the conjugate faults exhibit a V-shaped geometry with intersection angles of 60-75 degrees, which is 30-45 degrees greater than that predicted by the Anderson fault theory. In Tibet and Mongolia, geologic observations can rule out bookshelf faulting, distributed deformation, and temporal changes in stress state as explanations for the abnormal fault patterns. Instead, the GPS-determined velocity field across the conjugate fault zones indicate that the fault formation may have been related to Hagen-Poiseuille flow in map view involving the upper crust and possibly the whole lithosphere based on upper mantle seismicity in southern Tibet and basaltic volcanism in Mongolia. Such flow is associated with two coeval and parallel shear zones having opposite shear sense; each shear zone produce a set of Riedel shears, respectively, and together the Riedel shears exhibit the observed non-Andersonian conjugate strike-slip fault pattern. We speculate that the Hagen-Poiseuille flow across the lithosphere that hosts the conjugate strike-slip zones was produced by basal shear traction related to asthenospheric flow, which moves parallel and away from the indented segment of the collisional fronts. The inferred asthenospheric flow pattern below the conjugate strike-slip fault zones is consistent with the magnitude and orientations of seismic anisotropy observed across the Tibetan and Mongolian conjugate fault zones, suggesting a strong coupling between lithospheric deformation and asthenospheric flow. The laterally moving
-
Non-Andersonian conjugate strike-slip faults: Observations, theory, and tectonic implications
Energy Technology Data Exchange (ETDEWEB)
Yin, A [Department of Earth and Space Sciences and Institute of Geophysics and Planetary Physics, University of California, Los Angeles, Los Angeles, CA 90025-1567 (United States); Taylor, M H [Department of Geology, University of Kansas, 1475 Jayhawk Blvd., Lawrence, KS 66044 (United States)], E-mail: yin@ess.ucla.edu
2008-07-01
Formation of conjugate strike-slip faults is commonly explained by the Anderson fault theory, which predicts a X-shaped conjugate fault pattern with an intersection angle of {approx}30 degrees between the maximum compressive stress and the faults. However, major conjugate faults in Cenozoic collisional orogens, such as the eastern Alps, western Mongolia, eastern Turkey, northern Iran, northeastern Afghanistan, and central Tibet, contradict the theory in that the conjugate faults exhibit a V-shaped geometry with intersection angles of 60-75 degrees, which is 30-45 degrees greater than that predicted by the Anderson fault theory. In Tibet and Mongolia, geologic observations can rule out bookshelf faulting, distributed deformation, and temporal changes in stress state as explanations for the abnormal fault patterns. Instead, the GPS-determined velocity field across the conjugate fault zones indicate that the fault formation may have been related to Hagen-Poiseuille flow in map view involving the upper crust and possibly the whole lithosphere based on upper mantle seismicity in southern Tibet and basaltic volcanism in Mongolia. Such flow is associated with two coeval and parallel shear zones having opposite shear sense; each shear zone produce a set of Riedel shears, respectively, and together the Riedel shears exhibit the observed non-Andersonian conjugate strike-slip fault pattern. We speculate that the Hagen-Poiseuille flow across the lithosphere that hosts the conjugate strike-slip zones was produced by basal shear traction related to asthenospheric flow, which moves parallel and away from the indented segment of the collisional fronts. The inferred asthenospheric flow pattern below the conjugate strike-slip fault zones is consistent with the magnitude and orientations of seismic anisotropy observed across the Tibetan and Mongolian conjugate fault zones, suggesting a strong coupling between lithospheric deformation and asthenospheric flow. The laterally moving
-
Energy Technology Data Exchange (ETDEWEB)
Li, Nan-Nan; Zheng, Bing-Na [DSAPM Lab and PCFM Lab, Institute of Polymer Science, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275 (China); Lin, Jian-Tao [DSAPM Lab and PCFM Lab, Institute of Polymer Science, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275 (China); Guangdong Medical College, Dongguan 523808 (China); Zhang, Li-Ming, E-mail: ceszhlm@mail.sysu.edu.cn [DSAPM Lab and PCFM Lab, Institute of Polymer Science, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275 (China)
2014-01-01
New heparin–indomethacin conjugate with an ester linkage was prepared by the carbodiimide-mediated condensation reaction, and then characterized by FTIR and {sup 1}HNMR analyses. Due to its amphiphilic character, such a conjugate could self-aggregate into spherical nanoparticles in aqueous system, as confirmed by fluorescence spectroscopy, dynamic light scattering and transmission electron microscopy. By the in vitro drug release tests, the resultant conjugate nanoparticles were found to have a sustained and esterase-sensitive release behavior for conjugated indomethacin. In addition, the uptake of these conjugate nanoparticles into human nasopharyngeal carcinoma CNE1 cells was confirmed by fluorescence microscopy. - Highlights: • New heparin–indomethacin conjugate with an ester linkage was prepared. • Such a conjugate could self-aggregate into spherical nanoparticles in aqueous system. • The resultant conjugate nanoparticles exhibited an esterase-sensitive drug release behavior. • The resultant conjugate nanoparticles showed the cellular uptake ability in CNE1 cells.
-
Threshold couplings of phase-conjugate mirrors with two interaction regions.
Beli, M; Petrovi, M; Sandfuchs, O; Kaiser, F
1998-03-01
Using the grating-action method, we determine the threshold coupling strengths of three generic examples of phase-conjugate mirrors with two interaction regions: the cat conjugator, the mutually incoherent beam coupler, and the interconnected ring mirror.
-
/sup 125/I Radioimmunoassay of serum ursodeoxycholyl conjugates
Energy Technology Data Exchange (ETDEWEB)
Hill, A.; Ross, P.E.; Bouchier, I.A.D. (Ninewells Hospital and Medical School, Dundee (UK))
1983-02-07
A radioimmunoassay for serum ursodeoxycholic conjugates using an iodine-125 ligand has been developed. The bile acid was present in normal fasting serum (0.19 +- SD 0.19 ..mu..mol/l, n=24) and 2-hour post-prandial serum (0.8 +- SD 0.8 ..mu..mol/l, n=16). Gallstone patients undergoing oral ursodeoxycholic acid therapy had significantly higher post-prandial serum levels (21.5 +- SD 14.0 ..mu..mol/l, n=15) by radioimmunoassay. Gas liquid chromatography analysis indicated that in normal serum ursodeoxycholic acid was totally conjugated, whereas sera from gallstone patients contained a proportion as the free bile acid (10.2 +- SD 8.1 ..mu..mol/l, n=15). Following an oral dose of ursodeoxycholic acid, both unconjugated and conjugated forms of the bile acid appeared in the serum of healthy individuals.
-
International Nuclear Information System (INIS)
Khalil, Abdelouahed; Milochevitch, Christelle
2005-01-01
It is well known that vitamin E (α-tocopherol, α-toc) is a very efficient lipid soluble antioxidant and several studies showed its beneficial action in the prevention and reduction of atherosclerosis. However, some in vitro studies suggest a prooxidant role of vitamin E, which could occur under given circumstances. This study was thus designed to investigate the antioxidant vs. prooxidant effect of vitamin E with regards to LDL peroxidation induced under different oxidative stress conditions. LDL was enriched with α-tocopherol and different α-toc/LDL ratios were studied (8.0±2.5, 14.3±3.0, 33.3±3.7, 42.7±3.5 and 48.2±4.5 molecules of α-toc/LDL particle). Enriched and control LDL were oxidized by action of · OH and O 2 ·- free radicals produced by γ-radiolysis at different dose rates. Susceptibility of LDL to oxidation was examined by the measure of conjugated diene and TBARS formation as well as LDL endogenous α-toc disappearance. Increasing LDL α-toc concentration reduced the LDL susceptibility to oxidation and their oxidizability. α-toc disappearance rates were comprised between 43 and 8.3x10 -10 M s -1 and decreased with the radiation dose rate. Our results support an antioxidant role for α-tocopherol at high and low oxidative stress conditions
-
Energy Technology Data Exchange (ETDEWEB)
Khalil, Abdelouahed [Research Centre on Aging and Department of Medicine, University of Sherbrooke, Sherbrooke, QC, J1H 4C4 (Canada)]. E-mail: abdelouahed.khalil@usherbrooke.ca; Milochevitch, Christelle [Research Centre on Aging and Department of Medicine, University of Sherbrooke, Sherbrooke, QC, J1H 4C4 (Canada)
2005-02-01
It is well known that vitamin E ({alpha}-tocopherol, {alpha}-toc) is a very efficient lipid soluble antioxidant and several studies showed its beneficial action in the prevention and reduction of atherosclerosis. However, some in vitro studies suggest a prooxidant role of vitamin E, which could occur under given circumstances. This study was thus designed to investigate the antioxidant vs. prooxidant effect of vitamin E with regards to LDL peroxidation induced under different oxidative stress conditions. LDL was enriched with {alpha}-tocopherol and different {alpha}-toc/LDL ratios were studied (8.0{+-}2.5, 14.3{+-}3.0, 33.3{+-}3.7, 42.7{+-}3.5 and 48.2{+-}4.5 molecules of {alpha}-toc/LDL particle). Enriched and control LDL were oxidized by action of {sup {center_dot}}OH and O{sub 2}{sup {center_dot}}{sup -} free radicals produced by {gamma}-radiolysis at different dose rates. Susceptibility of LDL to oxidation was examined by the measure of conjugated diene and TBARS formation as well as LDL endogenous {alpha}-toc disappearance. Increasing LDL {alpha}-toc concentration reduced the LDL susceptibility to oxidation and their oxidizability. {alpha}-toc disappearance rates were comprised between 43 and 8.3x10{sup -10} M s{sup -1} and decreased with the radiation dose rate. Our results support an antioxidant role for {alpha}-tocopherol at high and low oxidative stress conditions.
-
Magnetic catechin-dextran conjugate as targeted therapeutic for pancreatic tumour cells.
Vittorio, Orazio; Voliani, Valerio; Faraci, Paolo; Karmakar, Biswajit; Iemma, Francesca; Hampel, Silke; Kavallaris, Maria; Cirillo, Giuseppe
2014-06-01
Catechin-dextran conjugates have recently attracted a lot of attention due to their anticancer activity against a range of cancer cells. Magnetic nanoparticles have the ability to concentrate therapeutically important drugs due to their magnetic-spatial control and provide opportunities for targeted drug delivery. Enhancement of the anticancer efficiency of catechin-dextran conjugate by functionalisation with magnetic iron oxide nanoparticles. Modification of the coating shell of commercial magnetic nanoparticles (Endorem) composed of dextran with the catechin-dextran conjugate. Catechin-dextran conjugated with Endorem (Endo-Cat) increased the intracellular concentration of the drug and it induced apoptosis in 98% of pancreatic tumour cells placed under magnetic field. The conjugation of catechin-dextran with Endorem enhances the anticancer activity of this drug and provides a new strategy for targeted drug delivery on tumour cells driven by magnetic field. The ability to spatially control the delivery of the catechin-dextran by magnetic field makes it a promising agent for further application in cancer therapy.
-
Fused thiophene-based conjugated polymers and their use in optoelectronic devices
Facchetti, Antonio; Marks, Tobin J.; Takai, Atsuro; Seger, Mark; Chen; , Zhihua
2017-07-18
The present teachings relate to polymeric compounds and their use as organic semiconductors in organic and hybrid optical, optoelectronic, and/or electronic devices such as photovoltaic cells, light emitting diodes, light emitting transistors, and field effect transistors. The disclosed compounds generally include as repeating units at least one annulated thienyl-vinylene-thienyl (TVT) unit and at least one other pi-conjugated unit. The annulated TVT unit can be represented by the formula: ##STR00001## where Cy.sup.1 and Cy.sup.2 can be a five- or six-membered carbocyclic ring. The annulated TVT unit can be optionally substituted at any available ring atom(s), and can be covalently linked to the other pi-conjugated unit via either the thiophene rings or the carbocyclic rings Cy.sup.1 and Cy.sup.2. The other pi-conjugated unit can be a conjugated linear linker including one or more unsaturated bonds, or a conjugated cyclic linker including one or more carbocyclic and/or heterocyclic rings.
-
Natural Antioxidants: Fascinating or Mythical Biomolecules?
Directory of Open Access Journals (Sweden)
Johannes Van Staden
2010-10-01
Full Text Available Research on the use, properties, characteristics and sources of antioxidants especially phenolic compounds, flavonoids, vitamins, synthetic chemicals and some micronutrients began in the late 18th century. Since then antioxidant research has received considerable attention and over a hundred thousand papers have been published on the subject. This has led to a rampant use of antioxidants in order to try to obtain and preserve optimal health. A number of nutraceuticals and food supplements are frequently fortified with synthetic or natural antioxidants. However, some research outcomes have led to the belief that antioxidants exist as mythical biomolecules. This review provides a critical evaluation of some common in vitro antioxidant capacity methods, and a discussion on the role and controversies surrounding non-enzymatic biomolecules, in particular phenolic compounds and non-phenolic compounds, in oxidative processes in an attempt of stemming the tidal wave that is threatening to swamp the concept of natural antioxidants.
-
Antioxidants for female subfertility.
Showell, Marian G; Mackenzie-Proctor, Rebecca; Jordan, Vanessa; Hart, Roger J
2017-07-28
A couple may be considered to have fertility problems if they have been trying to conceive for over a year with no success. This may affect up to a quarter of all couples planning a child. It is estimated that for 40% to 50% of couples, subfertility may result from factors affecting women. Antioxidants are thought to reduce the oxidative stress brought on by these conditions. Currently, limited evidence suggests that antioxidants improve fertility, and trials have explored this area with varied results. This review assesses the evidence for the effectiveness of different antioxidants in female subfertility. To determine whether supplementary oral antioxidants compared with placebo, no treatment/standard treatment or another antioxidant improve fertility outcomes for subfertile women. We searched the following databases (from their inception to September 2016) with no language or date restriction: Cochrane Gynaecology and Fertility Group (CGFG) specialised register, the Cochrane Central Register of Studies (CENTRAL CRSO), MEDLINE, Embase, PsycINFO, CINAHL and AMED. We checked reference lists of appropriate studies and searched for ongoing trials in the clinical trials registers. We included randomised controlled trials (RCTs) that compared any type, dose or combination of oral antioxidant supplement with placebo, no treatment or treatment with another antioxidant, among women attending a reproductive clinic. We excluded trials comparing antioxidants with fertility drugs alone and trials that only included fertile women attending a fertility clinic because of male partner infertility. Two review authors independently selected eligible studies, extracted the data and assessed the risk of bias of the included studies. The primary review outcome was live birth; secondary outcomes included clinical pregnancy rates and adverse events. We pooled studies using a fixed-effect model, and calculated odds ratios (ORs) with 95% confidence intervals (CIs) for the dichotomous
-
Antioxidants and the Comet assay.
Cemeli, Eduardo; Baumgartner, Adolf; Anderson, Diana
2009-01-01
It is widely accepted that antioxidants, either endogenous or from the diet, play a key role in preserving health. They are able to quench radical species generated in situations of oxidative stress, either triggered by pathologies or xenobiotics, and they protect the integrity of DNA from genotoxicants. Nevertheless, there are still many compounds with unclear or unidentified prooxidant/antioxidant activities. This is of concern since there is an increase in the number of compounds synthesized or extracted from vegetables to which humans might be exposed. Despite the well-established protective effects of fruit and vegetables, the antioxidant(s) responsible have not all been clearly identified. There might also be alternative mechanisms contributing to the protective effects for which a comprehensive description is lacking. In the last two decades, the Comet assay has been extensively used for the investigation of the effects of antioxidants and many reports can be found in the literature. The Comet assay, a relatively fast, simple, and sensitive technique for the analysis of DNA damage in all cell types, has been applied for the screening of chemicals, biomonitoring and intervention studies. In the present review, several of the most well-known antioxidants are considered. These include: catalase, superoxide dismutase, glutathione peroxidase, selenium, iron chelators, melatonin, melanin, vitamins (A, B, C and E), carotenes, flavonoids, isoflavones, tea polyphenols, wine polyphenols and synthetic antioxidants. Investigations showing beneficial as well as non-beneficial properties of the antioxidants selected, either at the in vitro, ex vivo or in vivo level are discussed.
-
Miranda, Jonatan; Arias, Noemi; Fernández-Quintela, Alfredo; del Puy Portillo, María
2014-04-01
Despite its benefits, conjugated linoleic acid (CLA) may cause side effects after long-term administration. Because of this and the controversial efficacy of CLA in humans, alternative biomolecules that may be used as functional ingredients have been studied in recent years. Thus, conjugated linolenic acid (CLNA) has been reported to be a potential anti-obesity molecule which may have additional positive effects related to obesity. According to the results reported in obesity, CLNA needs to be given at higher doses than CLA to be effective. However, because of the few studies conducted so far, it is still difficult to reach clear conclusions about the potential use of these CLNAs in obesity and its related changes (insulin resistance, dyslipidemia, or inflammation). Copyright © 2012 SEEN. Published by Elsevier Espana. All rights reserved.
-
Effect of TiO2 on conjugative transfer of RP4 plasmid
International Nuclear Information System (INIS)
Qian Di; Zhang Buchang; Yang Dong; Chen Zhaoli; Jin Min; Qiu Zhigang; Li Junwen
2013-01-01
Objective: To explore the effect and law of nano-titanium dioxide on the conjugative transfer of RP4 plasmid. Methods: Mating was conducted between Escherichia coli HB101 (RP4) and E. coli K12Rif in saline without stirring under certain conditions and the donor per recipient ratio was 1:1 constantly. The selective LB agar medium plates containing appropriate antibiotics were used to count the number of transconjugants and the conjugative transfer frequency. Results: Nano-titanium dioxide could promote the conjugative transfer of RP4. The nano-titanium dioxide concentration, bacterial concentration, mating temperature and mating time could affect the conjugative transfer of RP4. Conclusion: Nano-titanium dioxide can promote plasmid conjugal transfer in the liquid phase under certain conditions, which may pose a potential hazard to environmental and human health. (authors)
-
Yu, Songcheng; Yu, Fei; Zhang, Hongquan; Qu, Lingbo; Wu, Yongjun
2014-06-01
In this study, in order to find out a proper method for conjugation of enrofloxacin to label enzymes, two methods were compared and carbodiimide condensation was proved to be better. The results showed that the binding ratio of enrofloxacin and alkaline phosphatase (ALP) was 8:1 and that of enrofloxacin and horseradish peroxidase (HRP) was 5:1. This indicated that conjugate synthesized by carbodiimide condensation was fit for chemiluminescence enzyme immunoassay (CLEIA). Furthermore, data revealed that dialysis time was an important parameter for conjugation and 6 days was best. Buffer to dilute conjugate had little effect on CLEIA. The storage condition for conjugates was also studied and it was shown that the conjugate was stable at 4 °C with no additive up to 30 days. These data were valuable for establishing CLEIA to quantify enrofloxacin.
-
Directory of Open Access Journals (Sweden)
Garry Duthie
2012-01-01
Full Text Available Flavonoids are polyphenolic compounds with potential antioxidant activity via multiple reduction capacities. Oxidation of cellular lipids has been implicated in many diseases. Consequently, this study has assessed the ability of several dietary flavonoid aglycones to suppress lipid peroxidation of hepatic microsomes derived from rats deficient in the major lipid soluble antioxidant, dα-tocopherol. Antioxidant effectiveness was galangin > quercetin > kaempferol > fisetin > myricetin > morin > catechin > apigenin. However, none of the flavonoids were as effective as dα-tocopherol, particularly at the lowest concentrations used. In addition, there appears to be an important distinction between the in vitro antioxidant effectiveness of flavonoids and their ability to suppress indices of oxidation in vivo. Compared with dα-tocopherol, repletion of vitamin E deficient rats with quercetin, kaempferol, or myricetin did not significantly affect indices of lipid peroxidation and tissue damage. Direct antioxidant effect of flavonoids in vivo was not apparent probably due to low bioavailability although indirect redox effects through stimulation of the antioxidant response element cannot be excluded.
-
Dietary antioxidants for the athlete.
Atalay, Mustafa; Lappalainen, Jani; Sen, Chandan K
2006-06-01
Physical exercise induces oxidative stress and tissue damage. Although a basal level of reactive oxygen species (ROS) is required to drive redox signaling and numerous physiologic processes, excess ROS during exercise may have adverse implications on health and performance. Antioxidant nutrients may be helpful in that regard. Caution should be exercised against excess antioxidant supplements, however. This article presents a digest for sports practitioners. The following three recommendations are made: 1) it is important to determine the individual antioxidant need of each athlete performing a specific sport; 2) multinutrient preparations, as opposed to megadoses of any single form of nutrient, seem to be a more prudent path to choose; and 3) for outcomes of antioxidant supplementation, performance should not be the only criteria. Overall well being of the athlete, faster recovery, and minimization of injury time could all be affected by antioxidant therapy.
-
Combining CPT-conjugate neutrino channels at Fermilab
International Nuclear Information System (INIS)
Jansson, Andreas; Parke, Stephen; Saoulidou, Niki; Mena, Olga
2008-01-01
We explore an alternative strategy to determine the neutrino mass hierarchy by making use of possible future neutrino facilities at Fermilab. Here, we use CPT-conjugate neutrino channels, exploiting a ν μ beam from the NuMI beamline and a ν e beam from a beta-beam experimental setup. Both experiments are performed at approximately the same /L. We present different possible accelerator scenarios for the beta-beam neutrino setup and fluxes. This CPT-conjugate neutrino channel scenario can extract the neutrino mass hierarchy down to sin 2 2θ 13 ≅0.02.
-
Directory of Open Access Journals (Sweden)
Iqbal, Shahid
2010-09-01
Full Text Available The antioxidant potential of 100% and 80% methanol extracts from the seeds of three barley varieties (Jou 83, Jou 87 and Haider 93 was assessed. The extract yields from barley seeds ranged from 3.23% (Haider 93,100% methanol to 5.31% (Jou 83, 80% methanol. The total phenolic contents, DPPH radical scavenging activity (IC50 values and inhibition of linoleic acid oxidation of barley seed extracts (BSE were determined to be 88.1-145.7 mg/100g, 90.8-168.6 μg/mL and 62.6-74.6%, respectively. The antioxidant effectiveness of BSE was also assessed by stabilizing sunflower oil (SFO with BSE at a concentration of 600 ppm (oil weight basis. The stabilized (treated with extract and the control (without extract addition SFO samples were subjected to accelerated (oven heating at 60ºC for 30 days, 8 h heating cycle/day storage. These were analyzed at regular intervals for the extent of oxidative changes according to the measurements of their contents of peroxide value, para-anisidine value, conjugated dienes and conjugated trienes. Generally, the 80% methanol extract of barely seeds demonstrated better antioxidant action than the 100% methanol extract. The antioxidant activity of BSE was also found to be considerably varied among the varieties tested. The present results suggest that antioxidant extracts from barely seeds might be used to protect vegetable oils from oxidation.El potencial antioxidante de extractos de metanol al 100% y el 80% de semillas de tres variedades de cebada (Jou 83, Jou 87 y Haider 93 fue evaluada. El rendimiento de los extractos de las semillas de cebada vario desde un 3.23% (Haider, 100% methanol a un 5.31% (Jou 83, 80% metanol. El contenido total de fenoles, la actividad atrapadora del radical DPPH (valores IC50 y la inhibición de la oxidación del ácido linoleico de los extractos de semilla de cebada (BSE fueron 88.1-145.7 mg/100g, 90.8-168.6 μg/mL y 62.6- 74.6%, respectivamente. La efectividad antioxidante de BSE fue tambi
-
Homology among tet determinants in conjugative elements of streptococci
Energy Technology Data Exchange (ETDEWEB)
Smith, M.D.; Hazum, S.; Guild, W.R.
1981-10-01
A mutation to tetracycline sensitivity in a resistant strain of Streptococcus pneumoniae was shown by several criteria to be due to a point mutation in the conjugative o(cat-tet) element found in the chromosomes of strains derived from BM6001, a clinical strain resistant to tetracycline and chloramphenicol. Strains carrying the mutation were transformed back to tetracycline resistance with the high efficiency of a point marker by donor deoxyribonucleic acids from its ancestral strain and from nine other clinical isolates of pneumococcus and by deoxyribonucleic acids from Group D Streptococcus faecalis and Group B Streptococcus agalactiae strains that also carry conjugative tet elements in their chromosomes. It was not transformed to resistance by tet plasmid deoxyribonucleic acids from either gram-negative or gram-positive species, except for one that carried transposon TN916, the conjugative tet element present in the chromosomes of some S. faecalis strains. The results showed that the tet determinants in conjugative elements of several streptococcal species share a high degree of deoxyribonucleic acid sequence homology and suggested that they differ from other tet genes.
-
Parallel conjugate gradient algorithms for manipulator dynamic simulation
Fijany, Amir; Scheld, Robert E.
1989-01-01
Parallel conjugate gradient algorithms for the computation of multibody dynamics are developed for the specialized case of a robot manipulator. For an n-dimensional positive-definite linear system, the Classical Conjugate Gradient (CCG) algorithms are guaranteed to converge in n iterations, each with a computation cost of O(n); this leads to a total computational cost of O(n sq) on a serial processor. A conjugate gradient algorithms is presented that provide greater efficiency using a preconditioner, which reduces the number of iterations required, and by exploiting parallelism, which reduces the cost of each iteration. Two Preconditioned Conjugate Gradient (PCG) algorithms are proposed which respectively use a diagonal and a tridiagonal matrix, composed of the diagonal and tridiagonal elements of the mass matrix, as preconditioners. Parallel algorithms are developed to compute the preconditioners and their inversions in O(log sub 2 n) steps using n processors. A parallel algorithm is also presented which, on the same architecture, achieves the computational time of O(log sub 2 n) for each iteration. Simulation results for a seven degree-of-freedom manipulator are presented. Variants of the proposed algorithms are also developed which can be efficiently implemented on the Robot Mathematics Processor (RMP).
-
Target binding improves relaxivity in aptamer-gadolinium conjugates.
Bernard, Elyse D; Beking, Michael A; Rajamanickam, Karunanithi; Tsai, Eve C; Derosa, Maria C
2012-12-01
MRI contrast agents (CA) have been heavily used over the past several decades to enhance the diagnostic value of the obtained images. From a design perspective, two avenues to improve the efficacy of contrast agents are readily evident: optimization of magnetic properties of the CA, and optimization of the pharmacokinetics and distribution of the CA in the patient. Contrast agents consisting of DNA aptamer-gadolinium(III) conjugates provide a single system in which these factors can be addressed simultaneously. In this proof-of-concept study, the 15mer thrombin aptamer was conjugated to diethylenetriaminepentaacetic (DTPA) dianhydride to form a monoamide derivative of the linear open-chain chelate present in the commonly used contrast agent Magnevist(®). The stability of the conjugated DNA aptamer-DTPA-Gd(III) chelate in a transmetallation study using Zn(II) was found to be similar to that reported for DTPA-Gd(III). Relaxivity enhancements of 35 ± 4 and 20 ± 1 % were observed in the presence of thrombin compared to a control protein at fields of 9.4 and 1.5 T, respectively. The inclusion of spacers between the aptamer and the DTPA to eliminate possible steric effects was also investigated but not found to improve the relaxation enhancement achieved in comparison to the unaltered aptamer conjugate.
-
Phase conjugation with random fields and with deterministic and random scatterers
International Nuclear Information System (INIS)
Gbur, G.; Wolf, E.
1999-01-01
The theory of distortion correction by phase conjugation, developed since the discovery of this phenomenon many years ago, applies to situations when the field that is conjugated is monochromatic and the medium with which it interacts is deterministic. In this Letter a generalization of the theory is presented that applies to phase conjugation of partially coherent waves interacting with either deterministic or random weakly scattering nonabsorbing media. copyright 1999 Optical Society of America
-
HER2 specific delivery of methotrexate by dendrimer conjugated anti-HER2 mAb
International Nuclear Information System (INIS)
Shukla, Rameshwer; Thomas, Thommey P; Desai, Ankur M; Kotlyar, Alina; Park, Steve J; Baker, James R Jr
2008-01-01
Herceptin, a humanized monoclonal antibody that binds to human growth factor receptor-2 (HER2), was covalently attached to a fifth-generation (G5) polyamidoamine dendrimer containing the cytotoxic drug methotrexate. The specific binding and internalization of this conjugate labeled with FITC was clearly demonstrated in cell lines overexpressing HER2 by flow cytometry as well as confocal microscopic analysis. In addition, binding and uptake of antibody conjugated dendrimers was completely blocked by excess non-conjugated herceptin. The dendrimer conjugate was also shown to inhibit the dihydrofolate reductase with similar activity to methotrexate. Co-localization experiments with lysotracker red indicate that antibody conjugate, although internalized efficiently into cells, has an unusually long residence time in the lysosome. Somewhat lower cytotoxicity of the conjugate in comparison to free methotrexate was attributed to the slow release of methotrexate from the conjugate and its long retention in the lysosomal pocket
-
Farag, Mohamed A; Sakna, Sarah T; El-Fiky, Nabaweya M; Shabana, Marawan M; Wessjohann, Ludger A
2015-11-01
Bauhinia L. (Fabaceae) comprises ca. 300-350 plant species, many of which are traditionally used in folk medicine for their antidiabetic, antioxidant and anti-inflammatory effects. Bauhinia s.l. recently has been subdivided into 9 genera based on phylogenetic data: Bauhinia s.str., Barklya, Brenierea, Gigasiphon, Lysiphyllum, Phanera, Piliostigma, Schnella (American Phanera) and Tylosema. The aerial parts of 8 species corresponding to 5 genera were analyzed: Bauhinia forficata, Bauhinia variegata, B. variegata var. candida, Bauhinia galpinii, Schnella glabra, Piliostigma racemosa, Phanera vahlii and Lysiphyllum hookeri. Leaves and shoots were subjected to metabolite profiling via UHPLC-PDA-qTOF-MS coupled to multivariate data analyzes to identify compound compositional differences. A total of 90 metabolites were identified including polyphenols and fatty acids; flavonoid conjugates accounted for most of the metabolite variation observed. This study provides a comprehensive map of polyphenol composition in Bauhinia and phytochemical species aggregations are consistent with recent Bauhinia genus taxonomic relationship derived from phylogenetic studies. DPPH radical scavenging and α-glucosidase inhibitory assays were also performed to assess selected aspects of the antioxidant and antidiabetic potential for the examined species with respect to metabolite profiles. Copyright © 2015 Elsevier Ltd. All rights reserved.
-
Molecules with linear pi-conjugated pathways between all substituents : Omniconjugation
van der Veen, M.H.; Rispens, M.T; Jonkman, H.T.; Hummelen, J.C.
In this paper, omniconjugation is introduced as a topological phenomenon in pi-conjugated systems. Omniconjugated molecules are defined by the fact that they provide direct and fully pi-conjugated pathways between all subdstituents attached to them. Surprisingly, until now such topologies have never
-
Molecules with Linear π-Conjugated Pathways between All Substituents : Omniconjugation
Veen, Marleen H. van der; Rispens, Minze T.; Jonkman, Harry T.; Hummelen, Jan C.
2004-01-01
In this paper, omniconjugation is introduced as a topological phenomenon in π-conjugated systems. Omniconjugated molecules are defined by the fact that they provide direct and fully π-conjugated pathways between all substituents attached to them. Surprisingly, until now such topologies have never
-
Apak, Reşat; Güçlü, Kubilay; Ozyürek, Mustafa; Bektaşoğlu, Burcu; Bener, Mustafa
2010-01-01
Tests measuring the combined antioxidant effect of the nonenzymatic defenses in biological fluids may be useful in providing an index of the organism's capability to counteract reactive species known as pro-oxidants, resist oxidative damage, and combat oxidative stress-related diseases. The selected chromogenic redox reagent for the assay of human serum should be easily accessible, stable, selective, and respond to all types of biologically important antioxidants such as ascorbic acid, alpha-tocopherol, beta-carotene, reduced glutathione (GSH), uric acid, and bilirubin, regardless of chemical type or hydrophilicity. Our recently developed cupric reducing antioxidant capacity (CUPRAC) spectrophotometric method for a number of polyphenols and flavonoids using the copper(II)-neocuproine reagent in ammonium acetate buffer is now applied to a complete series of plasma antioxidants for the assay of total antioxidant capacity of serum, and the resulting absorbance at 450 nm is recorded either directly (e.g., for ascorbic acid, alpha-tocopherol, and glutathione) or after incubation at 50 degrees C for 20 min (e.g., for uric acid, bilirubin, and albumin), quantitation being made by means of a calibration curve. The lipophilic antioxidants, alpha-tocopherol and beta-carotene, are assayed in dichloromethane. Lipophilic antioxidants of serum are extracted with n-hexane from an ethanolic solution of serum subjected to centrifugation. Hydrophilic antioxidants of serum are assayed in the centrifugate after perchloric acid precipitation of proteins. The CUPRAC molar absorptivities, linear ranges, and TEAC (trolox equivalent antioxidant capacity) coefficients of the serum antioxidants are established, and the results are evaluated in comparison with the findings of the ABTS/TEAC reference method. The intra- and inter-assay coefficients of variation (CVs) are 0.7 and 1.5%, respectively, for serum. The CUPRAC assay proved to be efficient for glutathione and thiol-type antioxidants
-
Sequence-specific DNA alkylation by tandem Py-Im polyamide conjugates.
Taylor, Rhys Dylan; Kawamoto, Yusuke; Hashiya, Kaori; Bando, Toshikazu; Sugiyama, Hiroshi
2014-09-01
Tandem N-methylpyrrole-N-methylimidazole (Py-Im) polyamides with good sequence-specific DNA-alkylating activities have been designed and synthesized. Three alkylating tandem Py-Im polyamides with different linkers, which each contained the same moiety for the recognition of a 10 bp DNA sequence, were evaluated for their reactivity and selectivity by DNA alkylation, using high-resolution denaturing gel electrophoresis. All three conjugates displayed high reactivities for the target sequence. In particular, polyamide 1, which contained a β-alanine linker, displayed the most-selective sequence-specific alkylation towards the target 10 bp DNA sequence. The tandem Py-Im polyamide conjugates displayed greater sequence-specific DNA alkylation than conventional hairpin Py-Im polyamide conjugates (4 and 5). For further research, the design of tandem Py-Im polyamide conjugates could play an important role in targeting specific gene sequences. © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
-
Application of heat-balance integral method to conjugate thermal explosion
Directory of Open Access Journals (Sweden)
Novozhilov Vasily
2009-01-01
Full Text Available Conjugate thermal explosion is an extension of the classical theory, proposed and studied recently by the author. The paper reports application of heat-balance integral method for developing phase portraits for systems undergoing conjugate thermal explosion. The heat-balance integral method is used as an averaging method reducing partical differential equation problem to the set of first-order ordinary differential equations. The latter reduced problem allows natural interpretation in appropriately chosen phase space. It is shown that, with the help of heat-balance integral technique, conjugate thermal explosion problem can be described with a good accuracy by the set of non-linear first-order differential equations involving complex error function. Phase trajectories are presented for typical regimes emerging in conjugate thermal explosion. Use of heat-balance integral as a spatial averaging method allows efficient description of system evolution to be developed.
-
Lectins as carriers: preparation and purification of a concanavalin A-trypsin conjugate
International Nuclear Information System (INIS)
Shier, W.T.
1985-01-01
The scheme for the preparation and purification of Con A-trypsin demonstrates the presence of Con A in the conjugate by affinity purification and by hemagglutination. The presence of Con A was demonstrated in two additional ways. The conjugate was prepared using trypsin labeled with iodine 125 and unlabeled Con A. The resulting conjugate containing 42,800 cpm/mg protein was used to demonstrate prolonged retention of the conjugated trypsin in mouse footpads. The presence of trypsin was also demonstrated in the conjugate by affinity chromatography and by the esterase activity characteristic of trypsin with tosyl-L-arginine methyl ester as substrate. Con A-trypsin was also assayed for proteolytic activity with a macromolecular substrate azocasein. A comparison of proteolytic activities with these two substrates indicated that 50-80% of the proteolytic activity observed with the small substrate is retained with macromolecular substrates
-
Enhancing the Photoluminescence Emission of Conjugated MEH-PPV by Light Processing
Botiz, Ioan
2014-04-09
We show here that treatment of thin films of conjugated polymers by illumination with light leads to an increase of the intensity of their photoluminescence by up to 42%. The corresponding enhancement of absorbance was much less pronounced. We explain this significant enhancement of photoluminescence by a planarization of the conjugated polymer chains induced by photoexcitations even below the glass transition temperature, possibly due to an increased conjugation length. Interestingly, the photoluminescence remains at the enhanced level for more than 71 h after treatment of the films by illumination with light, likely due to the fact that below the glass transition temperature no restoring force could return the conjugated chains into their initial conformational state. © 2014 American Chemical Society.
-
Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action.
Bhavnani, Bhagu R; Stanczyk, Frank Z
2014-07-01
Oral conjugated equine estrogens (CEE) are the most used estrogen formulation for postmenopausal hormone therapy either alone or in combination with a progestin. CEE is most commonly used for the management of early menopausal symptoms such as hot flashes, vaginitis, insomnia, and mood disturbances. Additionally, if used at the start of the menopausal phase (age 50-59 years), CEE prevents osteoporosis and may in some women reduce the risk of cardiovascular disease (CVD) and Alzheimer's disease (AD). There appears to be a common mechanism through which estrogens can protect against CVD and AD. CEE is a natural formulation of an extract prepared from pregnant mares' urine. The product monogram lists the presence of only 10 estrogens consisting of the classical estrogens, estrone and 17β-estradiol, and a group of unique ring B unsaturated estrogens such as equilin and equilenin. The ring B unsaturated estrogens are formed by an alternate steroidogenic pathway in which cholesterol is not an obligatory intermediate. Both the route of administration and structure of these estrogens play a role in the overall pharmacology of CEE. In contrast to 17β-estradiol, ring B unsaturated estrogens express their biological effects mainly mediated by the estrogen receptor β and not the estrogen receptor α. All estrogen components of CEE are antioxidants, and some ring B unsaturated estrogens have several fold greater antioxidant activity than estrone and 17β-estradiol. The cardioprotective and neuroprotective effects of CEE appear to be, to some extent, due to its ability to prevent the formation of oxidized LDL and HDL, and by inhibiting or modulating some of the key proteases involved in programmed cell death (apoptosis) induced by the excess neurotransmitter glutamate and other neurotoxins. Selective combinations of ring B unsaturated estrogens have the potential of being developed as novel therapeutic agents for the prevention of cardiovascular disease and Alzheimer
-
Conjugation of Inulin Improves Anti-Biofilm Activity of Chitosan.
Zhang, Guiqiang; Liu, Jing; Li, Ruilian; Jiao, Siming; Feng, Cui; Wang, Zhuo A; Du, Yuguang
2018-05-04
Bacteria biofilm helps bacteria prevent phagocytosis during infection and increase resistance to antibiotics. Staphylococcus aureus is a Gram-positive pathogenic bacterium and is tightly associated with biofilm-related infections, which have led to great threat to human health. Chitosan, the only cationic polysaccharide in nature, has been demonstrated to have antimicrobial and anti-biofilm activities, which, however, require a relative high dosage of chitosan. Moreover, poor water solubility further restricts its applications on anti-infection therapy. Inulins are a group of polysaccharides produced by many types of plants, and are widely used in processed foods. Compared to chitosan, inulin is very soluble in water and possesses a mild antibacterial activity against certain pathogenic bacteria. In order to develop an effective strategy to treat biofilm-related infections, we introduce a method by covalent conjugation of inulin to chitosan. The physicochemical characterization of the inulin⁻chitosan conjugate was assayed, and the anti-biofilm activity was evaluated against S. aureus biofilm. The results indicated that, as compared to chitosan, this novel polysaccharide⁻polysaccharide conjugate significantly enhanced activities against S. aureus either in a biofilm or planktonic state. Of note, the conjugate also showed a broad spectrum anti-biofilm activity on different bacteria strains and low cellular toxicity to mammalian cells. These results suggested that chitosan conjugation of inulin was a viable strategy for treatment against biofilm-related infections. This finding may further spread the application of natural polysaccharides on treatments of infectious disease.
-
[Relationship between family variables and conjugal adjustment].
Jiménez-Picón, Nerea; Lima-Rodríguez, Joaquín-Salvador; Lima-Serrano, Marta
2018-04-01
To determine whether family variables, such as type of relationship, years of marriage, existence of offspring, number of members of family, stage of family life cycle, transition between stages, perceived social support, and/or stressful life events are related to conjugal adjustment. A cross-sectional and correlational study using questionnaires. Primary care and hospital units of selected centres in the province of Seville, Spain. Consecutive stratified sampling by quotas of 369 heterosexual couples over 18years of age, who maintained a relationship, with or without children, living in Seville. A self-report questionnaire for the sociodemographic variables, and the abbreviated version of the Dyadic Adjustment Scale, Questionnaire MOS Perceived Social Support, and Social Readjustment Rating Scale, were used. Descriptive and inferential statistics were performed with correlation analysis and multivariate regression. Statistically significant associations were found between conjugal adjustment and marriage years (r=-10: Pfamily life cycle (F=2.65; Pfamily life cycle stage (mature-aged stage) on conjugal adjustment (R2=.21; F=9.9; df=356; Prelationship. Copyright © 2017 Elsevier España, S.L.U. All rights reserved.
-
New preconditioned conjugate gradient algorithms for nonlinear unconstrained optimization problems
International Nuclear Information System (INIS)
Al-Bayati, A.; Al-Asadi, N.
1997-01-01
This paper presents two new predilection conjugate gradient algorithms for nonlinear unconstrained optimization problems and examines their computational performance. Computational experience shows that the new proposed algorithms generally imp lone the efficiency of Nazareth's [13] preconditioned conjugate gradient algorithm. (authors). 16 refs., 1 tab
-
Shwaery, G T; Vita, J A; Keaney, J F
1997-03-18
Exposure to estrogens reduces the risk for coronary artery disease and associated clinical events; however, the mechanisms responsible for these observations are not clear. Supraphysiological levels of estrogens act as antioxidants in vitro, limiting oxidation of low-density lipoprotein (LDL), an event implicated in atherogenesis. We investigated the conditions under which physiological concentrations of 17 beta-estradiol (E2) inhibit oxidative modification of LDL. Plasma incubated with E2 (0.1 to 100 nmol/L) for 4 hours yielded LDL that demonstrated a dose-related increase in resistance to oxidation by Cu2+ as measured by conjugated diene formation. This effect was dependent on plasma, because incubation of isolated LDL with E2 at these concentrations in buffered saline produced no effect on Cu(2+)-mediated oxidation. Incubation of plasma with E2 had no effect on LDL alpha-tocopherol content or cholesteryl ester hydroperoxide formation during the 4-hour incubation. Plasma incubation with [3H]E2 was associated with dose-dependent association of 3H with LDL. High-performance liquid chromatographic analysis of LDL derived from plasma incubated with [3H]E2 indicated that the majority of the associated species were not detectable as authentic E2 but as nonpolar forms of E2 that were susceptible to base hydrolysis consistent with fatty acid esterification of E2. Plasma-mediated association of E2 and subsequent antioxidant protection was inhibited by 5,5'-dithiobis(2-nitrobenzoic acid), an inhibitor of plasma acyltransferase activity. Exposure of LDL to physiological levels of E2 in a plasma milieu is associated with enhanced resistance to Cu(2+)-mediated oxidation and incorporation of E2 derivatives into LDL. This antioxidant capacity may be another means by which E2 limits coronary artery disease in women.
-
Orderings for conjugate gradient preconditionings
Ortega, James M.
1991-01-01
The effect of orderings on the rate of convergence of the conjugate gradient method with SSOR or incomplete Cholesky preconditioning is examined. Some results also are presented that help to explain why red/black ordering gives an inferior rate of convergence.
-
Antioxidants of edible mushrooms
Kozarski, Maja; Klaus, Anita; Jakovljevic, Dragica; Todorovic, Nina; Vunduk, Jovana; Petrović, Predrag; Niksic, Miomir; Vrvic, Miroslav M.; Griensven, Van Leo
2015-01-01
Oxidative stress caused by an imbalanced metabolism and an excess of reactive oxygen species (ROS) lead to a range of health disorders in humans. Our endogenous antioxidant defense mechanisms and our dietary intake of antioxidants potentially regulate our oxidative homeostasis. Numerous synthetic
-
alpha-Glucosidase-albumin conjugates: effect of chronic administration in mice
International Nuclear Information System (INIS)
Allen, T.M.; Murray, L.; Bhardwaj, D.; Poznansky, M.J.
1985-01-01
Enzyme albumin conjugates have been proposed as a means of increasing the efficacy of enzyme use in vivo and decreasing immune response to the enzyme. Particulate drug carriers, however, have a pronounced tendency to localize in the mononuclear phagocyte (reticuloendothelial) system. The authors have examined in mice the effect on phagocytic index, tissue distribution and organ size of continued administration of conjugates of alpha-glucosidase with either homologous or heterologous albumin. Mice received 10 X 2-mg injections of bovine serum albumin (BSA) or mouse serum albumin (MSA), either free, polymerized or conjugated with alpha-glucosidase. Experiments involving BSA had to be terminated before the end of the experiment because of anaphylaxis, but these reactions were less severe to the polymerized albumin than to free albumin. Free BSA, BSA polymer and BSA-enzyme conjugates all caused a decrease in phagocytic index after six injections. Mice receiving MSA showed no evidence of anaphylaxis, but mice receiving six or more injections of free MSA, MSA polymer or MSA-enzyme conjugate had significantly decreased phagocytic indices as compared to controls. Phagocytic indices had returned to normal by 7 days after the final injection. Tissue distribution of 125 I-labeled albumin preparations was determined in either naive or chronically injected mice
-
Directory of Open Access Journals (Sweden)
Đilas Sonja M.
2002-01-01
Full Text Available This paper attempts to lead the reader an understanding of what free radicals are and how they can form during lipid oxidation. Also, it provides some information out natural antioxidants (tocopherols and tocotrienols flavonoids, polyphenols, tannines, melanoidihes, carotenoids, ascorbates and the echanisms of their protection from radical damage. The sources of natural antioxidants are: oil seeds, teas, vegetables, fruits, spices and herbs.
-
Quantification of residual solvents in antibody drug conjugates using gas chromatography
Energy Technology Data Exchange (ETDEWEB)
Medley, Colin D., E-mail: medley.colin@gene.com [Genentech Inc., Small Molecule Pharmaceutical Sciences, 1 DNA Way, South San Francisco, CA 94080 (United States); Kay, Jacob [Research Pharmaceutical Services, 520 Virginia Dr. Fort, Washington, PA (United States); Li, Yi; Gruenhagen, Jason; Yehl, Peter; Chetwyn, Nik P. [Genentech Inc., Small Molecule Pharmaceutical Sciences, 1 DNA Way, South San Francisco, CA 94080 (United States)
2014-11-19
Highlights: • Sensitive residual solvents detection in ADCs. • 125 ppm QL for common conjugation solvents. • Generic and validatable method. - Abstract: The detection and quantification of residual solvents present in clinical and commercial pharmaceutical products is necessary from both patient safety and regulatory perspectives. Head-space gas chromatography is routinely used for quantitation of residual solvents for small molecule APIs produced through synthetic processes; however residual solvent analysis is generally not needed for protein based pharmaceuticals produced through cultured cell lines where solvents are not introduced. In contrast, antibody drug conjugates and other protein conjugates where a drug or other molecule is covalently bound to a protein typically use solvents such as N,N-dimethylacetamide (DMA), N,N‑dimethylformamide (DMF), dimethyl sulfoxide (DMSO), or propylene glycol (PG) to dissolve the hydrophobic small molecule drug for conjugation to the protein. The levels of the solvent remaining following the conjugation step are therefore important to patient safety as these parental drug products are introduced directly into the patients bloodstream. We have developed a rapid sample preparation followed by a gas chromatography separation for the detection and quantification of several solvents typically used in these conjugation reactions. This generic method has been validated and can be easily implemented for use in quality control testing for clinical or commercial bioconjugated products.
-
Emulsifying properties of maillard conjugates produced from sodium caseinate and locust bean gum
Directory of Open Access Journals (Sweden)
F. A. Perrechil
2014-06-01
Full Text Available Emulsifying properties of sodium caseinate -locust bean gum Maillard conjugates produced at different temperatures (54 - 96 ºC, protein/polysaccharide ratios (0.3 - 1.0 and reaction times (1 - 24 hours were evaluated. Conjugate formation was confirmed by formation of color and high molecular weight fractions and the decrease of the αs- and β-casein bands. The emulsions stabilized by Maillard conjugates showed good stability. The mean droplet diameter (d32 tended to decrease with the increase of incubation time and temperature, except at extreme conditions (24 hours and 90 ºC or 96 ºC when the partial degradation of the conjugates was probably favored, resulting in phase separation of emulsions. The emulsion viscosity decreased with the increase in the protein/polysaccharide ratio and with the degradation of the conjugates. The conditions used in the experimental design made the optimization of the conjugate production viable, which showed greater emulsifier properties than the pure protein under acid conditions.
-
International Nuclear Information System (INIS)
Jalvandi, Javid; White, Max; Gao, Yuan; Truong, Yen Bach; Padhye, Rajiv; Kyratzis, Ilias Louis
2017-01-01
A range of biodegradable drug-nanofibres composite mats have been reported as drug delivery systems. However, their main disadvantage is the rapid release of the drug immediately after application. This paper reports an improved system based on the incorporation of drug conjugated-chitosan into polyvinyl alcohol (PVA) nanofibers. The results showed that controlled release of levofloxacin (LVF) could be achieved by covalently binding LVF to low molecular weight chitosan (CS) via a cleavable amide bond and then blending the conjugated CS with polyvinyl alcohol (PVA) nanofibres prior to electrospinning. PVA/LVF and PVA-CS/LVF nanofibres were fabricated as controls. The conjugated CS-LVF was characterized by FTIR, DSC, TGA and 1 H NMR. Scanning electron microscopy (SEM) showed that the blended CS-PVA nanofibres had a reduced fibre diameter compared to the controls. Drug release profiles showed that burst release was decreased from 90% in the control PVA/LVF electrospun mats to 27% in the PVA/conjugated CS-LVF mats after 8 h in phosphate buffer at 37 °C. This slower release is due to the cleavable bond between LVF and CS that slowly hydrolysed over time at neutral pH. The results indicate that conjugation of the drug to the polymer backbone is an effective way of minimizing burst release behaviour and achieving sustained release of the drug, LVF. - Highlights: • A novel drug delivery system for controlled release of drug was designed. • Composite PVA/conjugated CS-LVF nanofibres was fabricated by electrospinning. • Conjugated chitosan and composite nanofibres were characterized by various techniques. • Release profiles of drug were significantly improved in composite nanofibres containing drug conjugated chitosan.
-
Energy Technology Data Exchange (ETDEWEB)
Jalvandi, Javid, E-mail: Javid.jlv@gmail.com [CSIRO, Manufacturing Flagship, Bayview Ave, Clayton, Victoria 3168 (Australia); School of Fashion and Textiles, College of Design and Social Context, RMIT University, 25 Dawson Street, Brunswick, Victoria 3056 (Australia); White, Max, E-mail: tamrak@bigpond.com [School of Fashion and Textiles, College of Design and Social Context, RMIT University, 25 Dawson Street, Brunswick, Victoria 3056 (Australia); Gao, Yuan, E-mail: Yuan.Gao@csiro.au [CSIRO, Manufacturing Flagship, Bayview Ave, Clayton, Victoria 3168 (Australia); Truong, Yen Bach, E-mail: Yen.truong@csiro.au [CSIRO, Manufacturing Flagship, Bayview Ave, Clayton, Victoria 3168 (Australia); Padhye, Rajiv, E-mail: rajiv.padhye@rmit.edu.au [School of Fashion and Textiles, College of Design and Social Context, RMIT University, 25 Dawson Street, Brunswick, Victoria 3056 (Australia); Kyratzis, Ilias Louis, E-mail: Louis.kyratzis@csiro.au [CSIRO, Manufacturing Flagship, Bayview Ave, Clayton, Victoria 3168 (Australia)
2017-04-01
A range of biodegradable drug-nanofibres composite mats have been reported as drug delivery systems. However, their main disadvantage is the rapid release of the drug immediately after application. This paper reports an improved system based on the incorporation of drug conjugated-chitosan into polyvinyl alcohol (PVA) nanofibers. The results showed that controlled release of levofloxacin (LVF) could be achieved by covalently binding LVF to low molecular weight chitosan (CS) via a cleavable amide bond and then blending the conjugated CS with polyvinyl alcohol (PVA) nanofibres prior to electrospinning. PVA/LVF and PVA-CS/LVF nanofibres were fabricated as controls. The conjugated CS-LVF was characterized by FTIR, DSC, TGA and {sup 1}H NMR. Scanning electron microscopy (SEM) showed that the blended CS-PVA nanofibres had a reduced fibre diameter compared to the controls. Drug release profiles showed that burst release was decreased from 90% in the control PVA/LVF electrospun mats to 27% in the PVA/conjugated CS-LVF mats after 8 h in phosphate buffer at 37 °C. This slower release is due to the cleavable bond between LVF and CS that slowly hydrolysed over time at neutral pH. The results indicate that conjugation of the drug to the polymer backbone is an effective way of minimizing burst release behaviour and achieving sustained release of the drug, LVF. - Highlights: • A novel drug delivery system for controlled release of drug was designed. • Composite PVA/conjugated CS-LVF nanofibres was fabricated by electrospinning. • Conjugated chitosan and composite nanofibres were characterized by various techniques. • Release profiles of drug were significantly improved in composite nanofibres containing drug conjugated chitosan.
-
Grafting functional antioxidants on highly crosslinked polyethylene
Al-Malaika, S.; Riasat, S.; Lewucha, C.
2016-05-01
The problem of interference of antioxidants, such as hindered phenols, with peroxide-initiated crosslinking of polyethylene was addressed through the use of functional (reactive) graftable antioxidants (g-AO). Reactive derivatives of hindered phenol and hindered amine antioxidants were synthesised, characterised and used to investigate their grafting reactions in high density polyethylene; both non-crosslinked (PE) and highly peroxide-crosslinked (PEXa). Assessment of the extent of in-situ grafting of the antioxidants, their retention after exhaustive solvent extraction in PE and PEXa, and the stabilising performance of the grafted antioxidants (g-AO) in the polymer were examined and benchmarked against conventionally stabilised crosslinked & non-crosslinked polyethylene. It was shown that the functional antioxidants graft to a high extent in PEXa, and that the level of interference of the g-AOs with the polymer crosslinking process was minimal compared to that of conventional antioxidants which bear the same antioxidant function. The much higher level of retention of the g-AOs in PEXa after exhaustive solvent extraction, compared to that of the corresponding conventional antioxidants, accounts for their superior long-term thermal stabilising performance under severe extractive conditions.
-
International Nuclear Information System (INIS)
Rekova, M.; Jedinakova-Krizova, V.
2010-01-01
The aim of the study of labeling of ligand-antibody conjugates was to find optimal conditions of preparing of these conjugates and appropriate radioactivity of selected nuclide for applications in nuclear medicine. Conjugation of the γ-immunoglobulin G (human or bovine IgG, polyclonal antibodies) and bifunctional chelating agent, diethylenetriaminepentaacetic acid dianhydride (cDTPAA), was carried out. Various values of the cDTPAA/antibody ratio, the weight concentration of polyclonal or monoclonal antibodies (MEM-97) and buffers were used. Further, the labeling conditions of the DTPA-IgG conjugate by radionuclides 90 Y and 177 Lu were optimized, and the labeling yield and the conjugation ratio of prepared radionuclide-DTPA-IgG conjugates was determined. Optimal incubation time of the immunoglobulin conjugation was obtained at 30 min from mixing of individual components. The labeling yield of radionuclide-DTPA-antibody conjugate higher than 95% was achieved. Higher values of conjugation ratio of radionuclide-DTPA-antibody conjugate were achieved in 0.1 mol L -1 carbonate buffer, pH 8.5, and the 0.1 mol L -1 carbonate buffer is suitable for studied conjugation systems. This study showed that the labeling yield as well as the conjugation ratio of tested systems depend on the amount of antibody substance, bifunctional chelating agent/antibody molar ratio and pH value of the buffer used. (author)
-
Molecularly precise dendrimer-drug conjugates with tunable drug release for cancer therapy.
Zhou, Zhuxian; Ma, Xinpeng; Murphy, Caitlin J; Jin, Erlei; Sun, Qihang; Shen, Youqing; Van Kirk, Edward A; Murdoch, William J
2014-10-06
The structural preciseness of dendrimers makes them perfect drug delivery carriers, particularly in the form of dendrimer-drug conjugates. Current dendrimer-drug conjugates are synthesized by anchoring drug and functional moieties onto the dendrimer peripheral surface. However, functional groups exhibiting the same reactivity make it impossible to precisely control the number and the position of the functional groups and drug molecules anchored to the dendrimer surface. This structural heterogeneity causes variable pharmacokinetics, preventing such conjugates to be translational. Furthermore, the highly hydrophobic drug molecules anchored on the dendrimer periphery can interact with blood components and alter the pharmacokinetic behavior. To address these problems, we herein report molecularly precise dendrimer-drug conjugates with drug moieties buried inside the dendrimers. Surprisingly, the drug release rates of these conjugates were tailorable by the dendrimer generation, surface chemistry, and acidity. © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
-
Two novel plasma diagnostic tools: fiber sensors and phase conjugation
International Nuclear Information System (INIS)
Jahoda, F.C.
1985-01-01
A rapidly developing technology (single-mode optical fiber sensors) and recent fundamental research in nonlinear optics (phase conjugation) both offer opportunities for novel plasma diagnostics. Single-mode fiber sensors can replace electrical wire probes for current and magnetic field measurements with advantages in voltage insulation requirements, electromagnetic noise immunity, much greater bandwidth, and some configuration flexibility. Faraday rotation measurements through fibers wound on the ZT-40M RFP have demonstrated quantitative results, but competing linear birefringence effects still hinder independent interpretation. Twisted fiber may solve this problem. Optical phase conjugation (in which a phase reversed copy of a laser beam is generated) allows real time distortion corrections in laser diagnostics. Self-pumped phase conjugation in BaTiO 3 improves the quality of phase conjugation imagery and greatly simplifies experimentation directed toward plasma diagnostics. Our initial applications are a) time-differential refractometry with high spatial resolution and b) intracavity absorption Zeeman spectroscopy
-
Excitons in conjugated polymers: Do we need a paradigma change?
Energy Technology Data Exchange (ETDEWEB)
Beenken, Wichard J.D. [Department of Theoretical Physics I, Ilmenau University of Thechnology (Germany)
2009-12-15
We have previously shown that both, polymer conformation and dynamics are crucial for the exciton transport in conjugated polymers. Thereby we found that the usual Foerster-type hopping transfer model - even if one applies the line-dipole approximation - falls short in one crucial aspect: the nature of the sites the excitons are transferred between is still unclear. We found that the simple model of spectroscopic units defined as segments of the polymer chains separated by structural defects breaking the {pi}-conjugation is only justified for chemical defects like hydrogenated double bonds, or extreme gauche (90 ) torsions between the monomers. Both defects are far too rare in a well-prepared conjugated polymer to explain the mean spectroscopic-unit length of typically 6-7 monomers. Meanwhile, also the concept of dynamical formation of the spectroscopic units, we had previously suggested, has also failed. Thus the question of a paradigma change concerning the exciton transport in conjugated polymers appears on the agenda. (Abstract Copyright [2009], Wiley Periodicals, Inc.)
-
DEFF Research Database (Denmark)
Houen, G.; Olsen, D.T.; Hansen, P.R.
2003-01-01
A solid-phase conjugation method utilizing carrier protein bound to an ion exchange matrix was developed. Ovalbumin was adsorbed to an anion exchange matrix using a batch procedure, and the immobilized protein was then derivatized with iodoacetic acid N-hydroxysuccinimid ester. The activated......, and immunization experiments with the eluted conjugates showed that the more substituted conjugates gave rise to the highest titers of glutathione antibodies. Direct immunization with the conjugates adsorbed to the ion exchange matrix was possible and gave rise to high titers of glutathione antibodies. Conjugates...... of ovalbumin and various peptides were prepared in a similar manner and used for production of peptide antisera by direct immunization with the conjugates bound to the ion exchanger. Advantages of the method are its solid-phase nature, allowing fast and efficient reactions and intermediate washings...
-
Conjugated Polymers as Actuators: Modes of Actuation
DEFF Research Database (Denmark)
Skaarup, Steen
2004-01-01
The physical and chemical properties of conjugated polymers often depend very strongly on the degree of doping with anions or cations. The movement of ions in and out of the polymer matrix as it is redox cycled is also accompanied by mechanical changes. Both the volume and the stiffness can exhibit...... significant differences between the oxidized and reduced states. These effects form the basis of the use of conjugated polymers as actuators (or “artificial muscles”) controllable by a small (1-10 V) voltage. Three basic modes of actuation (bending, linear extension and stiffness change) have been proposed...
-
Conjugated polymers as actuators: modes of actuation
DEFF Research Database (Denmark)
Skaarup, Steen
2007-01-01
The physical and chemical properties of conjugated polymers often depend very strongly on the degree of doping with anions or cations. The movement of ions in and out of the polymer matrix as it is redox cycled is also accompanied by mechanical changes. Both the volume and the stiffness can exhibit...... significant differences between the oxidized and reduced states. These effects form the basis of the use of conjugated polymers as actuators (or “artificial muscles”) controllable by a small (1-10 V) voltage. Three basic modes of actuation (bending, linear extension and stiffness change) have been proposed...
-
Functionalized conjugated polyelectrolytes design and biomedical applications
Wang, Shu
2014-01-01
Functionalized Conjugated Polyelectrolytes presents a comprehensive review of these polyelectrolytes and their biomedical applications. Basic aspects like molecular design and optoelectronic properties are covered in the first chapter. Emphasis is placed on the various applications including sensing (chemical and biological), disease diagnosis, cell imaging, drug/gene delivery and disease treatment. This book explores a multi-disciplinary topic of interest to researchers working in the fields of chemistry, materials, biology and medicine. It also offers an integrated perspective on both basic research and application issues. Functionalized conjugated polyelectrolyte materials, which have already drawn considerable interest, will become a major new direction for biomedicine development.
-
Persistence Mechanisms of Conjugative Plasmids
DEFF Research Database (Denmark)
Bahl, Martin Iain; Hansen, Lars H.; Sørensen, Søren Johannes
2009-01-01
Are plasmids selfish parasitic DNA molecules or an integrated part of the bacterial genome? This chapter reviews the current understanding of the persistence mechanisms of conjugative plasmids harbored by bacterial cells and populations. The diversity and intricacy of mechanisms affecting the suc...
-
Sanmartín-Suárez, Carolina; Soto-Otero, Ramón; Sánchez-Sellero, Inés; Méndez-Álvarez, Estefanía
2011-01-01
Dimethyl sulfoxide is an amphiphilic compound whose miscibility with water and its ability to dissolve lipophilic compounds make it an appreciated solvent in biomedical research. However, its reported antioxidant properties raise doubts about its use as a solvent in evaluating new antioxidants. The goal of this investigation was to evaluate its antioxidant properties and carry out a comparative study on the antioxidant properties of some known neuroprotective antioxidants in the presence and absence of dimethyl sulfoxide. The antioxidant properties of dimethyl sulfoxide were studied in rat brain homogenates by determining its ability to reduce both lipid peroxidation (TBARS formation) and protein oxidation (increase in protein carbonyl content and decrease in free thiol content) induced by ferrous chloride/hydrogen peroxide. Its ability to reduce the production of hydroxyl radicals by 6-hydroxydopamine autoxidation was also estimated. The same study was also performed with three known antioxidants (α-phenyl-N-tert-butylnitrone; 2-methyl-2-nitrosopropane; 5,5-dimethyl-1-pyrroline N-oxide) in the presence and absence of dimethyl sulfoxide. Our results showed that dimethyl sulfoxide is able to reduce both lipid peroxidation and protein carbonyl formation induced by ferrous chloride/hydrogen peroxide in rat brain homogenates. It can also reduce the production of hydroxyl radicals during 6-hydroxydopamine autoxidation. However, it increases the oxidation of protein thiol groups caused by ferrous chloride/hydrogen peroxide in rat brain homogenate. Despite the here reported antioxidant and pro-oxidant properties of dimethyl sulfoxide, the results obtained with α-phenyl-N-tert-butylnitrone, 2-methyl-2-nitrosopropane, and 5,5-dimethyl-1-pyrroline N-oxide corroborate the antioxidant properties attributed to these compounds and support the potential use of dimethyl sulfoxide as a solvent in the study of the antioxidant properties of lipophilic compounds. Dimethyl sulfoxide
-
International Nuclear Information System (INIS)
Mansour, S.Z.; EI-Tawil, G.A.; Mostafa, M.
2009-01-01
Ginger is a dietary natural product, which has antioxidant and anti carcinogenic properties. Its chemo prevention effect against hepato carcinogenesis induced by diethylnitrosamine (DEN) in gamma-irradiated rats was studied, in the present study. Rats were randomly divided into 7 groups. Group I served as control. Group 2 exposed to gamma-rays alone (4 fractionated doses; 3 Gy/ week up to a total dose of 12 Gy). Group 3 received a single intraperitoneal (ip) injection of DEN/ week at a dose of 30 mg/ Kg body wt consecutive for 10 weeks. Group 4 administered orally five days a week with ginger (50 mg/ 100 g body wt) there exposed to gamma-rays. Group 5 pre treated with ginger then injected with DEN. Group 6 injected with DEN and then exposed to gamma-rays. Group 7 pre treated with ginger then injected with DEN pre-irradiation. Our findings demonstrate gamma-irradiation and/ or DEN caused significant decrease in superoxide dismutase (SOD) and catalase (Cat) activities and glutathione (GSH) content in both blood and liver tissue. Also, they increase plasma and liver lipid peroxidation (LP), plasma alfa fetoprotein (AFP) and liver conjugated diene (CD). Orally administration of ginger to animals ameliorates such changes. It could be concluded that ginger has antioxidant and anti carcinogenic properties might protect against hepato carcinoma and gamma-radiation
-
Antioxidant Activity from Various Tomato Processing
Directory of Open Access Journals (Sweden)
Retno Sri Iswari
2016-04-01
Full Text Available Tomato is one of the high antioxidant potential vegetables. Nowadays, there are many techniques of tomato processings instead of fresh consumption, i.e. boiled, steamed, juiced and sauteed. Every treatment of cooking will influence the chemical compound inside the fruits and the body's nutrition intake. It is important to conduct the research on antioxidant compound especially lycopene, β-carotene, vitamin C, α-tocopherol, and its activity after processing. This research has been done using the experimental method. Tomatoes were cooked into six difference ways, and then it was extracted using the same procedure continued with antioxidant measurement. The research results showed that steaming had promoted the higher antioxidant numbers (lycopene. α-tocopherol, β-carotene and vitamin C and higher TCA and antioxidant activities in the tomatoes than other processings. It was indicated that steaming was the best way to enhance amount, capacity and activities of antioxidants of the tomatoes.
-
Stochastic Spectral and Conjugate Descent Methods
Kovalev, Dmitry
2018-02-11
The state-of-the-art methods for solving optimization problems in big dimensions are variants of randomized coordinate descent (RCD). In this paper we introduce a fundamentally new type of acceleration strategy for RCD based on the augmentation of the set of coordinate directions by a few spectral or conjugate directions. As we increase the number of extra directions to be sampled from, the rate of the method improves, and interpolates between the linear rate of RCD and a linear rate independent of the condition number. We develop and analyze also inexact variants of these methods where the spectral and conjugate directions are allowed to be approximate only. We motivate the above development by proving several negative results which highlight the limitations of RCD with importance sampling.
-
Stochastic Spectral and Conjugate Descent Methods
Kovalev, Dmitry; Gorbunov, Eduard; Gasanov, Elnur; Richtarik, Peter
2018-01-01
The state-of-the-art methods for solving optimization problems in big dimensions are variants of randomized coordinate descent (RCD). In this paper we introduce a fundamentally new type of acceleration strategy for RCD based on the augmentation of the set of coordinate directions by a few spectral or conjugate directions. As we increase the number of extra directions to be sampled from, the rate of the method improves, and interpolates between the linear rate of RCD and a linear rate independent of the condition number. We develop and analyze also inexact variants of these methods where the spectral and conjugate directions are allowed to be approximate only. We motivate the above development by proving several negative results which highlight the limitations of RCD with importance sampling.
-
Celik, Saliha Esin; Ozyürek, Mustafa; Güçlü, Kubilay; Apak, Reşat
2010-06-15
Antioxidants are health beneficial compounds that can protect cells and macromolecules (e.g., fats, lipids, proteins, and DNA) from the damage of reactive oxygen species (ROS). Solvent effect is a crucial parameter on the chemical behaviour of antioxidant compounds but there has been limited information regarding its role on antioxidant capacity and its assays. Therefore, the present study was undertaken to investigate the total antioxidant capacity (TAC) of some certain lipophilic and hydrophilic antioxidants, measured in different solvent media such as ethanol (EtOH) (100%), methanol (MeOH) (100%), methanol/water (4:1, v/v), methanol/water (1:1, v/v), dichloromethane (DCM)/EtOH (9:1, v/v). The cupric reducing antioxidant capacity (CUPRAC) values of selected antioxidants were experimentally reported in this work as trolox equivalent antioxidant capacity (TEAC), and compared to those found by reference TAC assays, i.e., 2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)/persulphate (ABTS/persulphate) and ferric reducing antioxidant power (FRAP) methods. The TAC values of synthetic mixtures of antioxidants were experimentally measured as trolox equivalents and compared to those theoretically found by making use of the principle of additivity of absorbances assuming no chemical interaction between the mixture constituents. Possible synergistic (e.g., BHT and BHA in DCM/EtOH) or antagonistic behaviours of these synthetic mixtures were investigated in relation to solvent selection.
-
Swain, Shasank S; Paidesetty, Sudhir K; Padhy, Rabindra N
2017-03-01
To develop 6 conjugate agents of the moribund antibiotic sulfamethoxazole (SMZ) joined to 6 individual monoterpenes, followed by protocols of medicinal chemistry as potent antibacterials, against multidrug resistant (MDR) human gruesome pathogenic bacteria. Antibacterial activities of the proposed conjugates were ascertained by the 'prediction of activity spectra of substances' (PASS) program. Drug-likeness parameters and toxicity profiles of conjugates were standardized with the Lipinski rule of five, using cheminformatic tools, Molsoft, molinspiration, OSIRIS and ProTox. Antibacterial activities of individual chemicals and conjugates were examined by targeting the bacterial folic acid biosynthesis enzyme, dihydropteroate synthases (DHPSs) of bacteria, Bacillus anthracis, Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae and Mycobacterium tuberculosis, with 3D structures of DHPSs from protein data bank. According to the PASS program, biological spectral values of conjugate-2, conjugate-5 and conjugate-6 were ascertained effective with 'probably active' or 'Pa' value > 0.5, for anti-infective and antituberculosic activities. Using molecular docking against 5 cited bacterial DHPSs, effective docking scores of 6 monoterpenes in the specified decreasing order (kcal/mol): -9.72 (eugenol against B. anthracis), -9.61 (eugenol against S. pneumoniae), -9. 42 (safrol, against B. anthracis), -9.39 (thymol, against M. tuberculosis), -9.34 (myristicin, against S. pneumoniae) and -9.29 (thymol, against B. anthracis); whereas the lowest docking score of SMZ was -8.46kcal/mol against S. aureus DHPS. Similarly, effective docking scores of conjugates were as specified (kcal/mol.): -10.80 (conjugate-4 consisting SMZ+safrol, against M. tuberculosis), -10.78 (conjugate-5 consisting SMZ+thymol, against M. tuberculosis), -10.60 (conjugate-5 against B. anthracis), -10.26 (conjugate-2 consisting SMZ+ eugenol, against M. tuberculosis), -10.25 (conjugate-5, against S
-
Uptake of Single-Walled Carbon Nanotubes Conjugated with DNA by Microvascular Endothelial Cells
Directory of Open Access Journals (Sweden)
Joseph Harvey
2012-01-01
Full Text Available Single-walled carbon nanotubes (SWCNTs have been proposed to have great therapeutic potential. SWCNTs conjugated with drugs or genes travel in the systemic circulation to reach target cells or tissues following extravasation from microvessels although the interaction between SWCNT conjugates and the microvascular endothelial cells (ECs remains unknown. We hypothesized that SWCNT-DNA conjugates would be taken up by microvascular ECs and that this process would be facilitated by SWCNTs compared to facilitation by DNA alone. ECs were treated with various concentrations of SWCNT-DNA-FITC conjugates, and the uptake and intracellular distribution of these conjugates were determined by a confocal microscope imaging system followed by quantitative analysis of fluorescence intensity. The uptake of SWCNT-DNA-FITC conjugates (2 μg/mL by microvascular ECs was significantly greater than that of DNA-FITC (2 μg/mL, observed at 6 hrs after treatment. For the intracellular distribution, SWCNT-DNA-FITC conjugates were detected in the nucleus of ECs, while DNA-FITC was restricted to the cytoplasm. The fluorescence intensity and distribution of SWCNTs were concentration and time independent. The findings demonstrate that SWCNTs facilitate DNA delivery into microvascular ECs, thus suggesting that SWCNTs serving as drug and gene vehicles have therapeutic potential.
-
Several Guaranteed Descent Conjugate Gradient Methods for Unconstrained Optimization
Directory of Open Access Journals (Sweden)
San-Yang Liu
2014-01-01
Full Text Available This paper investigates a general form of guaranteed descent conjugate gradient methods which satisfies the descent condition gkTdk≤-1-1/4θkgk2 θk>1/4 and which is strongly convergent whenever the weak Wolfe line search is fulfilled. Moreover, we present several specific guaranteed descent conjugate gradient methods and give their numerical results for large-scale unconstrained optimization.
-
Conjugation of curcumin onto alginate enhances aqueous solubility and stability of curcumin.
Dey, Soma; Sreenivasan, K
2014-01-01
Curcumin is a potential drug for various diseases including cancer. Prime limitations associated with curcumin are low water solubility, rapid hydrolytic degradation and poor bioavailability. In order to redress these issues we developed Alginate-Curcumin (Alg-Ccm) conjugate which was characterized by FTIR and (1)H NMR spectroscopy. The conjugate self-assembled in aqueous solution forming micelles with an average hydrodynamic diameter of 459 ± 0.32 nm and negative zeta potential. The spherical micelles were visualized by TEM. The critical micelle concentration (CMC) of Alg-Ccm conjugate was determined. A significant enhancement in the aqueous solubility of curcumin was observed upon conjugation with alginate. Formation of micelles improved the stability of curcumin in water at physiological pH. The cytotoxic activity of Alg-Ccm was quantified by MTT assay using L-929 fibroblast cells and it was found to be potentially cytotoxic. Hence, Alg-Ccm could be a promising drug conjugate as well as a nanosized delivery vehicle. Copyright © 2013 Elsevier Ltd. All rights reserved.
-
Comparison of linoleic and conjugated linoleic acids in enzymatic acidolysis of tristearin
DEFF Research Database (Denmark)
Yang, Tiankui; Xu, Xuebing; Li, L.T.
2001-01-01
tristearin (SSS) and linoleic (L) or conjugated linoleic (cL) acids (1:6, mol/mol). The other was between tristearin and the mixture of linoleic and conjugated linoleic acids (1:3:3, mol/mol/mol). Acyl incorporation and migration together with triacylglycerol composition of the products were monitored...... with gas chromatography, pancreatic lipase hydrolysis, and high performance liquid chromatography. Both acyl incorporation and migration of linoleic acid were faster than those of conjugated linoleic acid. At 5 h reaction, there were 13.0% LLL, 46.5% LSL, 27.7% LSS, and 5.6% SSS in the product for a system...... between tristearin and linoleic acid; whereas there were 2.4% cLcLcL, 10.4% cLScL, 50.9% cLSS, and 36.2% SSS in the product for a system between tristearin and conjugated linoleic acid. The results suggest that linoleic acid was more reactive than conjugated linoleic acid in the enzymatic acidolysis...
-
Partitioning of selected antioxidants in mayonnaise
DEFF Research Database (Denmark)
Jacobsen, Charlotte; Schwarz, K.; Stockmann, H.
1999-01-01
This study examined partitioning of alpha-, beta-, and gamma- tocopherol and six polar antioxidants (Trolox, ferulic acid, caffeic acid, propyl gallate, gallic acid, and catechin) in mayonnaise. Partitioning of antioxidants between different phases was determined after separation of mayonnaise...... acid and catechin) to 83% (Trolox). Accordingly, proportions of 6% (Trolox) to 80% (gallic acid and catechin) were found in the aqueous phase. Similar trends were observed after dialysis. After ultracentrifugation, large proportions of polar antioxidants were found in the "emulsion phase...... by either (a) centrifugation + ultracentrifugation or (b) centrifugation + dialysis. Antioxidants partitioned in accordance with their chemical structure and polarity: Tocopherols were concentrated in the oil phase (93-96%), while the proportion of polar antioxidants in the oil phase ranged from 0% (gallic...
-
Antioxidant Protection in Blood against Ionising Radiation
International Nuclear Information System (INIS)
Bognar, G.; Meszaros, G.; Koteles, G. J.
2001-01-01
Full text: The quantities of the antioxidants in the human blood are important indicators of health status. The routine determinations of activities/capacities of antioxidant compounds would be of great importance in assessing individual sensitivities against oxidative effects. We have investigated the sensitivities of those antioxidant elements against various doses of ionising radiation tested by the RANDOX assays. Our results show dose-dependent decreases of antioxidant activities caused by the different doses. The total antioxidant status value linearly decreased up to 1 Gy, but further increase of dose (2 Gy) did not influence the respective values although the test system still indicated their presence. It means that the human blood retains 60-70% of its total antioxidant capacity. Radiation induced alterations of the antioxidant enzymes: glutathione peroxidase and superoxide dismutase have been also investigated. The activities of glutathione peroxidase and superoxide dismutase decreased linearly upon the effects of various doses of ionising radiation till 1 Gy. Between 1 and 2 Gy only further mild decreases could be detected. In this case the human blood retained 40-60% of these two antioxidant enzymes. These observations suggest either the limited response of antioxidant system against ionising radiation, or the existence of protection system of various reactabilities. (author)
-
Effects of transferrin conjugated multi-walled carbon nanotubes in lung cancer delivery
Energy Technology Data Exchange (ETDEWEB)
Singh, Rahul Pratap [Department of Pharmacology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005 (India); Sharma, Gunjan [Genotoxicology and Cancer Biology Lab, Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi 221005 (India); Sonali [Department of Pharmacology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005 (India); Singh, Sanjay [Department of Pharmaceutics, Indian Institute of Technology (BHU), Varanasi 221005 (India); Patne, Shashikant C.U. [Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005 (India); Pandey, Bajarangprasad L. [Department of Pharmacology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005 (India); Koch, Biplob, E-mail: kochbiplob@gmail.com [Genotoxicology and Cancer Biology Lab, Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi 221005 (India); Muthu, Madaswamy S., E-mail: muthubits@rediffmail.com [Department of Pharmaceutics, Indian Institute of Technology (BHU), Varanasi 221005 (India); Department of Pharmacology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005 (India)
2016-10-01
The aim of this study was to develop multi-walled carbon nanotubes (MWCNT) which were covalently conjugated with transferrin by carbodiimide chemistry and loaded with docetaxel as a model drug for effective treatment of lung cancer in comparison with the commercial docetaxel injection (Docel™). D-Alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) was used as amphiphilic surfactant to improve the aqueous dispersity and biocompatibility of MWCNT. Human lung cancer cells (A549 cells) were employed as an in-vitro model to access cellular uptake, cytotoxicity, cellular apoptosis, cell cycle analysis, and reactive oxygen species (ROS) of the docetaxel/coumarin-6 loaded MWCNT. The cellular uptake results of transferrin conjugated MWCNT showed higher efficiency in comparison with free C6. The IC{sub 50} values demonstrated that the transferrin conjugated MWCNT could be 136-fold more efficient than Docel™ after 24 h treatment with the A549 cells. Flow cytometry analysis confirmed that cancerous cells appeared significantly (P < 0.05) in the sub-G1 phase for transferrin conjugated MWCNT in comparison with Docel™. Results of transferrin conjugated MWCNT have showed better efficacy with safety than Docel™. - Highlights: • It shows the development of transferrin conjugated MWCNT formulation of DTX for the effective treatment of lung cancer. • Evaluated the cellular uptake, cytotoxicity, cellular apoptosis, cell cycle, and ROS level of the DTX/C6 loaded MWCNT. • The IC{sub 50} values demonstrated that the transferrin conjugated MWCNT could be 136-fold more effective than Docel™. • Safety of the DTX formulations were studied by the measurements of ALP, LDH and total protein count levels in BAL fluid. • Results of transferrin conjugated MWCNT have showed better efficacy with safety than Docel™ in lung cancer delivery.
-
Effects of transferrin conjugated multi-walled carbon nanotubes in lung cancer delivery
International Nuclear Information System (INIS)
Singh, Rahul Pratap; Sharma, Gunjan; Sonali; Singh, Sanjay; Patne, Shashikant C.U.; Pandey, Bajarangprasad L.; Koch, Biplob; Muthu, Madaswamy S.
2016-01-01
The aim of this study was to develop multi-walled carbon nanotubes (MWCNT) which were covalently conjugated with transferrin by carbodiimide chemistry and loaded with docetaxel as a model drug for effective treatment of lung cancer in comparison with the commercial docetaxel injection (Docel™). D-Alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) was used as amphiphilic surfactant to improve the aqueous dispersity and biocompatibility of MWCNT. Human lung cancer cells (A549 cells) were employed as an in-vitro model to access cellular uptake, cytotoxicity, cellular apoptosis, cell cycle analysis, and reactive oxygen species (ROS) of the docetaxel/coumarin-6 loaded MWCNT. The cellular uptake results of transferrin conjugated MWCNT showed higher efficiency in comparison with free C6. The IC_5_0 values demonstrated that the transferrin conjugated MWCNT could be 136-fold more efficient than Docel™ after 24 h treatment with the A549 cells. Flow cytometry analysis confirmed that cancerous cells appeared significantly (P < 0.05) in the sub-G1 phase for transferrin conjugated MWCNT in comparison with Docel™. Results of transferrin conjugated MWCNT have showed better efficacy with safety than Docel™. - Highlights: • It shows the development of transferrin conjugated MWCNT formulation of DTX for the effective treatment of lung cancer. • Evaluated the cellular uptake, cytotoxicity, cellular apoptosis, cell cycle, and ROS level of the DTX/C6 loaded MWCNT. • The IC_5_0 values demonstrated that the transferrin conjugated MWCNT could be 136-fold more effective than Docel™. • Safety of the DTX formulations were studied by the measurements of ALP, LDH and total protein count levels in BAL fluid. • Results of transferrin conjugated MWCNT have showed better efficacy with safety than Docel™ in lung cancer delivery.
-
2013-03-28
...-Up Exclusive License: Photosensitizing Antibody-Fluorophore Conjugates for Photoimmunotherapy AGENCY...-205-2010/0-US-01), and entitled ``Photosensitizing Antibody- Fluorophore Conjugates,'' to Aspyrian... invention. The field of use may be limited to ``use of photosensitizing antibody-fluorophore conjugate by...
-
Single-particle tracking of quantum dot-conjugated prion proteins inside yeast cells
Energy Technology Data Exchange (ETDEWEB)
Tsuji, Toshikazu; Kawai-Noma, Shigeko [Department of Biomolecular Engineering, Graduate School of Biosciences and Biotechnology, Tokyo Institute of Technology, B56, 4259 Nagatsuta, Midori-ku, Yokohama 226-8501 (Japan); Pack, Chan-Gi [Cellular Informatics Laboratory, RIKEN Advanced Science Institute, Wako-shi, Saitama 351-0198 (Japan); Terajima, Hideki [Department of Biomolecular Engineering, Graduate School of Biosciences and Biotechnology, Tokyo Institute of Technology, B56, 4259 Nagatsuta, Midori-ku, Yokohama 226-8501 (Japan); Yajima, Junichiro; Nishizaka, Takayuki [Department of Physics, Gakushuin University, 1-5-1 Mejiro, Toshima-ku, Tokyo 171-8588 (Japan); Kinjo, Masataka [Laboratory of Molecular Cell Dynamics, Graduate School of Life Sciences, Hokkaido University, Sapporo 001-0021 (Japan); Taguchi, Hideki, E-mail: taguchi@bio.titech.ac.jp [Department of Biomolecular Engineering, Graduate School of Biosciences and Biotechnology, Tokyo Institute of Technology, B56, 4259 Nagatsuta, Midori-ku, Yokohama 226-8501 (Japan)
2011-02-25
Research highlights: {yields} We develop a method to track a quantum dot-conjugated protein in yeast cells. {yields} We incorporate the conjugated quantum dot proteins into yeast spheroplasts. {yields} We track the motions by conventional or 3D tracking microscopy. -- Abstract: Yeast is a model eukaryote with a variety of biological resources. Here we developed a method to track a quantum dot (QD)-conjugated protein in the budding yeast Saccharomyces cerevisiae. We chemically conjugated QDs with the yeast prion Sup35, incorporated them into yeast spheroplasts, and tracked the motions by conventional two-dimensional or three-dimensional tracking microscopy. The method paves the way toward the individual tracking of proteins of interest inside living yeast cells.
-
Single-particle tracking of quantum dot-conjugated prion proteins inside yeast cells
International Nuclear Information System (INIS)
Tsuji, Toshikazu; Kawai-Noma, Shigeko; Pack, Chan-Gi; Terajima, Hideki; Yajima, Junichiro; Nishizaka, Takayuki; Kinjo, Masataka; Taguchi, Hideki
2011-01-01
Research highlights: → We develop a method to track a quantum dot-conjugated protein in yeast cells. → We incorporate the conjugated quantum dot proteins into yeast spheroplasts. → We track the motions by conventional or 3D tracking microscopy. -- Abstract: Yeast is a model eukaryote with a variety of biological resources. Here we developed a method to track a quantum dot (QD)-conjugated protein in the budding yeast Saccharomyces cerevisiae. We chemically conjugated QDs with the yeast prion Sup35, incorporated them into yeast spheroplasts, and tracked the motions by conventional two-dimensional or three-dimensional tracking microscopy. The method paves the way toward the individual tracking of proteins of interest inside living yeast cells.
-
Nonlinear piezoelectricity in PZT ceramics for generating ultrasonic phase conjugate waves
Yamamoto; Kokubo; Sakai; Takagi
2000-03-01
We have succeeded in the generation of acoustic phase conjugate waves with nonlinear PZT piezoelectric ceramics and applied them to ultrasonic imaging systems. Our aim is to make a phase conjugator with 100% efficiency. For this purpose, it is important to clarify the mechanism of acoustic phase conjugation through nonlinear piezoelectricity. The process is explained by the parametric interaction via the third-order nonlinear piezoelectricity between the incident acoustic wave at angular frequency omega and the pump electric field at 2 omega. We solved the coupling equations including the third-ordered nonlinear piezoelectricity and theoretically derived the amplitude efficiency of the acoustic phase conjugation. We compared the efficiencies between the theoretical and experimental values for PZT ceramics with eight different compositions. Pb[(Zn1/3Nb2/3)(1 - x)Tix]O3 (X = 0.09, PZNT91/9) piezoelectric single crystals have been investigated for high-performance ultrasonic transducer application, because these have large piezoelectric constants, high electrical-mechanical coupling factors and high dielectric constants. We found that they have third-order nonlinear piezoelectric constants much larger than PZT and are hopeful that the material as a phase conjugator has over 100% efficiency.
-
Storage Conditions of Conjugated Reagents Can Impact Results of Immunogenicity Assays
Directory of Open Access Journals (Sweden)
Robert J. Kubiak
2016-01-01
Full Text Available Consistent performance of anti-drug antibody (ADA assays through all stages of clinical development is critical for the assessment of immunogenicity and interpretation of PK, PD, safety, and efficacy. The electrochemiluminescent assays commonly employed for ADA measurement use drug conjugated with ruthenium and biotin to bind ADA in samples. Here we report an association between high nonspecific ADA responses in certain drug-naïve individuals and the storage buffer of the conjugated reagents used in a monoclonal antibody ADA assay. Ruthenylated reagents stored in phosphate-buffered saline (PBS buffer had increased levels of aggregate and produced variable and high baseline responses in some subjects. Reagents stored in a histidine-sucrose buffer (HSB had lower aggregate levels and produced low sample responses. In contrast to PBS, conjugated reagents formulated in HSB remained low in aggregate content and in sample response variability after 5 freeze/thaw cycles. A reagent monitoring control (RMC serum was prepared for the real-time evaluation of conjugated reagent quality. Using appropriate buffers for storage of conjugated reagents together with RMCs capable of monitoring of reagent aggregation status can help ensure consistent, long-term performance of ADA methods.
-
Intracellular trafficking of new anticancer therapeutics: antibody–drug conjugates
Kalim, Muhammad; Chen, Jie; Wang, Shenghao; Lin, Caiyao; Ullah, Saif; Liang, Keying; Ding, Qian; Chen, Shuqing; Zhan, Jinbiao
2017-01-01
Antibody–drug conjugate (ADC) is a milestone in targeted cancer therapy that comprises of monoclonal antibodies chemically linked to cytotoxic drugs. Internalization of ADC takes place via clathrin-mediated endocytosis, caveolae-mediated endocytosis, and pinocytosis. Conjugation strategies, endocytosis and intracellular trafficking optimization, linkers, and drugs chemistry present a great challenge for researchers to eradicate tumor cells successfully. This inventiveness of endocytosis and intracellular trafficking has given considerable momentum recently to develop specific antibodies and ADCs to treat cancer cells. It is significantly advantageous to emphasize the endocytosis and intracellular trafficking pathways efficiently and to design potent engineered conjugates and biological entities to boost efficient therapies enormously for cancer treatment. Current studies illustrate endocytosis and intracellular trafficking of ADC, protein, and linker strategies in unloading and also concisely evaluate practically applicable ADCs. PMID:28814834
-
Intracellular trafficking of new anticancer therapeutics: antibody-drug conjugates.
Kalim, Muhammad; Chen, Jie; Wang, Shenghao; Lin, Caiyao; Ullah, Saif; Liang, Keying; Ding, Qian; Chen, Shuqing; Zhan, Jinbiao
2017-01-01
Antibody-drug conjugate (ADC) is a milestone in targeted cancer therapy that comprises of monoclonal antibodies chemically linked to cytotoxic drugs. Internalization of ADC takes place via clathrin-mediated endocytosis, caveolae-mediated endocytosis, and pinocytosis. Conjugation strategies, endocytosis and intracellular trafficking optimization, linkers, and drugs chemistry present a great challenge for researchers to eradicate tumor cells successfully. This inventiveness of endocytosis and intracellular trafficking has given considerable momentum recently to develop specific antibodies and ADCs to treat cancer cells. It is significantly advantageous to emphasize the endocytosis and intracellular trafficking pathways efficiently and to design potent engineered conjugates and biological entities to boost efficient therapies enormously for cancer treatment. Current studies illustrate endocytosis and intracellular trafficking of ADC, protein, and linker strategies in unloading and also concisely evaluate practically applicable ADCs.
-
Repercussions of imprisonment for conjugal violence: discourses of men
Directory of Open Access Journals (Sweden)
Anderson Reis de Sousa
Full Text Available ABSTRACT Objective: to know the consequences that men experience related to incarceration by conjugal violence. Methods: qualitative study on 20 men in jail and indicted in criminal processes related to conjugal violence in a Court specialized in Family and Domestic Violence against women. The interviews were classified based on Collective Subject Discourse method, using NVIVO(r software. Results: the collective discourse shows that the experience of preventive imprisonment starts a process of family dismantling, social stigma, financial hardship and psycho-emotional symptoms such as phobia, depression, hypertension, and headaches. Conclusion: due to the physical, mental and social consequences of the conjugal violence-related imprisonment experience, it is urgent to look carefully into the somatization process as well as to the prevention strategies regarding this process.
-
Conjugate heat transfer of laminar film condensation along a horizontal plate
Energy Technology Data Exchange (ETDEWEB)
Lee, Euk Soo [Pusan National Univesity, Busan (Korea, Republic of)
2006-03-15
This paper proposes appropriate conjugate parameters and dimensionless temperatures to analysis the conjugate problem of heat conduction in solid wall coupled with laminar film condensation flow adjacent to horizontal flat plate. An efficient methods for some fluids are proposed for its solution. The momentum and energy balance equations are reduced to a nonlinear system of ordinary differential equations with four parameters: the Prandtl number, Pr, Modified Jacob number, Ja{sup *}/Pr, defined by an overall temperature difference, a property ratio {radical}{rho}{sub {iota}}{mu}{sub {iota}} {radical}{rho}{sub {upsilon}}{mu}{sub {upsilon}} and the conjugate parameter {zeta}. The obtained similarity solution reveals the effect of the conjugate parameter, and the results are compared with the simplified solution. The variations of the heat transfer rates as well as the interface temperature and frictions along the plate are shown explicitly.
-
Albumin–Polymer–Drug Conjugates: Long Circulating, High Payload Drug Delivery Vehicles
DEFF Research Database (Denmark)
Smith, Anton Allen Abbotsford; Zuwala, Kaja; Pilgram, Oliver
2016-01-01
Albumin is an exquisite tool of nature used in biomedicine to achieve long blood residence time for drugs, but the payload it can carry is typically limited to one molecule per protein. In contrast, synthetic macromolecular prodrugs contain multiple copies of drugs per polymer chain but offer only...... a marginal increase in the circulation lifetime of the drugs. We combine the benefits of the two platforms and at the same time overcome their respective limitations. Specifically, we develop the synthesis of albumin–polymer–drug conjugates to obtain long circulating, high payload drug delivery vehicles....... In vivo data validate that albumin endows the conjugate with a blood residence time similar to that of the protein and well exceeding that of the polymer. Therapeutic activity of the conjugates is validated using prodrugs of panobinostat, an HIV latency reversal agent, in which case the conjugates matched...
-
DNA-templated antibody conjugation for targeted drug delivery to cancer cells
DEFF Research Database (Denmark)
Liu, Tianqiang
2016-01-01
-templated organic synthesis due to the wide existence of the 3-histidine cluster in most wild-type proteins. In this thesis, three projects that relate to targeted drug delivery to cancer cells based on the DTPC method is described. The first project was a delivery system which uses transferrin as the targeting....... The study shows that DNA is a highly useful tool for the assembly of proteins with potential therapeutic applications. The DNA-templated protein conjugation shows a promising application in constructing antibody-toxin conjugates or antibody-drug conjugates. In addition, DNA strands used for antibody...... either antibody engineering or special expression systems and are both time and labor consuming. To avoid the drawbacks caused by conventional chemical modification and recombinant methodologies, an ideal site specific conjugation technique would use natural amino acid residues to the protein by a new...
-
The mitochondrial toxicity of cysteine-S-conjugates: Studies with pentachlorobutadienyl-L-cysteine
International Nuclear Information System (INIS)
Wallin, A.
1990-01-01
Nephrotoxic cysteine conjugates, arising from mercapturate biosynthesis, can perturb the mitochondrial membrane potential and calcium homeostasis in renal epithelial cells. Activation of these cysteine conjugates to reactive species by mitochondrial β-lyases results in covalent binding and mitochondrial damage. PCBC and related cysteine conjugates inhibit ADP-stimulated respiration in mitochondria respiring on alpha-ketoglutrate/malate and succinate indicating that both dehydrogenases may be targets. The respiratory inhibition is blocked by aminooxyacetic acid, an inhibitor of the β-lyase. Hence, metabolic activation is required implying that covalent binding of reactive intermediates may be important to the mitochondrial injury. Binding of 35 S-fragments has been found for 5 conjugates with varying degrees of mitochondrial toxicity. PCBC is more lipophilic and has a higher affinity for cellular membranes than other cysteine conjugates. PCBC rapidly depolarizes the inner membrane potential resulting in an inhibition of mitochondrial oxidative phosphorylation and calcium upon sequestration. Consequently, mitochondria and renal epithelial cells exposed to PCBC show a sudden release of calcium upon exposure to PCBC which is followed by a later increase in state 4 respiration leading to an inhibition of oxidative phosphorylation. The primary effect of other cysteine conjugates is an inhibition of the dehydrogenases, thus inhibiting state 3 respiration
-
High Relaxivity Gadolinium Hydroxypyridonate-Viral Capsid Conjugates: Nano-sized MRI Contrast Agents
Energy Technology Data Exchange (ETDEWEB)
Meux, Susan C.; Datta, Ankona; Hooker, Jacob M.; Botta, Mauro; Francis, Matthew B.; Aime, Silvio; Raymond, Kenneth N.
2007-08-29
High relaxivity macromolecular contrast agents based on the conjugation of gadolinium chelates to the interior and exterior surfaces of MS2 viral capsids are assessed. The proton nuclear magnetic relaxation dispersion (NMRD) profiles of the conjugates show up to a five-fold increase in relaxivity, leading to a peak relaxivity (per Gd{sup 3+} ion) of 41.6 mM{sup -1}s{sup -1} at 30 MHz for the internally modified capsids. Modification of the exterior was achieved through conjugation to flexible lysines, while internal modification was accomplished by conjugation to relatively rigid tyrosines. Higher relaxivities were obtained for the internally modified capsids, showing that (1) there is facile diffusion of water to the interior of capsids and (2) the rigidity of the linker attaching the complex to the macromolecule is important for obtaining high relaxivity enhancements. The viral capsid conjugated gadolinium hydroxypyridonate complexes appear to possess two inner-sphere water molecules (q = 2) and the NMRD fittings highlight the differences in the local motion for the internal ({tau}{sub RI} = 440 ps) and external ({tau}{sub RI} = 310 ps) conjugates. These results indicate that there are significant advantages of using the internal surface of the capsids for contrast agent attachment, leaving the exterior surface available for the installation of tissue targeting groups.
-
Protti, Michele; Gualandi, Isacco; Mandrioli, Roberto; Zappoli, Sergio; Tonelli, Domenica; Mercolini, Laura
2017-09-05
Goji berries and derived products represent a relevant source of micronutrients, most of which are natural antioxidants and contribute to the high nutritional quality of these fruits. Three brands of dried goji berries have been analysed by a multidisciplinary approach to get an insight into both their content of selected antioxidants and their antioxidant capacity (AC). The former goal has been achieved by developing a liquid chromatographic method coupled to mass spectrometry and combined to a fast solid phase extraction. Several significant representative antioxidant compounds belonging to the following classes: flavonoids, flavan-3-ols, phenolic acids, amino acids and derivatives, and carotenoids have been taken into account. Quercetin and rutin were found to be the predominant flavonoids, chlorogenic acid was the most abundant phenolic acid and zeaxanthin was the major carotenoid. The AC of the goji berries has been evaluated by four analytical methods in order to estimate the contributions of different reactions involved in radicals scavenging. In particular, AC has been determined using 3 standardised methods (DPPH, ABTS, ORAC) and a recently proposed electrochemical method, which measures the scavenging activity of antioxidants towards OH radicals generated both by hydrogen peroxide photolysis and the Fenton reaction. The results obtained from chemical composition and antioxidant capacity assays confirm the high nutritional and commercial value of goji berries and highlight that the three brands do not exhibit significant differences. Copyright © 2017 Elsevier B.V. All rights reserved.
-
ANTIOXIDANT POTENCY OF WATER KEFIR
Directory of Open Access Journals (Sweden)
Muneer Alsayadi M.S.
2013-06-01
Full Text Available Reactive oxygen species (ROS have strong relationship with several diseases. Many fermented foods were reported to be important sources for antioxidant compounds. Antioxidant activity of water kefir never reported in the scientific literature. The objective of this study was to detect and investigate the antioxidant potency of water kefir. Water kefir was prepared by fermentation of sugar solution with kefir grains for 24h. Antioxidant activity of fresh water kefir drink and its extract with (0.125–5 mg/ml was evaluated using 2,2,-diphenyl-1-pricrylhydrozyl (DPPH scavenging method, and inhibition of ascorbate autoxidation and the reducing power of water kefir were determined, Butylated hydroxyanisole (BHA and ascorbic acid were used for comparison. Water kefir demonstrated great ability to DPPH scavenging ranged (9.88-63.17%. And inhibit ascorbate oxidation by (6.08-25.57% increased in consequent with concentration raising. These results prime to conclude that water kefir could be promisor source of natural antioxidants with good potency in health developing.
-
Self-Assembly and Crystallization of Conjugated Block Copolymers
Davidson, Emily Catherine
This dissertation demonstrates the utility of molecular design in conjugated polymers to create diblock copolymers that robustly self-assemble in the melt and confine crystallization upon cooling. This work leverages the model conjugated polymer poly(3-(2'-ethyl)hexylthiophene) (P3EHT), which features a branched side chain, resulting in a dramatically reduced melting temperature (Tm 80°C) relative to the widely-studied poly(3-hexylthiophene) (P3HT) (Tm 200°C). This reduced melting temperature permits an accessible melt phase, without requiring that the segregation strength (chiN) be dramatically increased. Thus, diblock copolymers containing P3EHT demonstrate robust diblock copolymer self-assembly in the melt over a range of compositions and morphologies. Furthermore, confined crystallization in the case of both glassy (polystyrene (PS) matrix block) and soft (polymethylacrylate (PMA) matrix block) confinement is studied, with the finding that even in soft confinement, crystallization is constrained within the diblock microdomains. This success demonstrates the strategy of leveraging molecular design to decrease the driving force for crystallization as a means to achieving robust self-assembly and confined crystallization in conjugated block copolymers. Importantly, despite the relatively flexible nature of P3EHT in the melt, the diblock copolymer phase behavior appears to be significantly impacted by the stiffness (persistence length of 3 nm) of the P3EHT chain compared to the coupled amorphous blocks (persistence length 0.7 nm). In particular, it is shown that the synthesized morphologies are dominated by a very large composition window for lamellar geometries (favored at high P3EHT volume fractions); cylindrical geometries are favored when P3EHT is the minority fraction. This asymmetry of the composition window is attributed to impact of conformational asymmetry (the difference in chain stiffness, as opposed to shape) between conjugated and amorphous blocks
-
Zhou, Zhan; Zhang, Jing; Zhang, Yan; Ma, Guanghui; Su, Zhiguo
2016-01-20
Conventional preparation strategies for antibody-drug conjugates (ADCs) result in heterogeneous products with various molecular sizes and species. In this study, we developed a homogeneous preparation strategy by site-specific conjugation of the anticancer drug with an antibody fragment. The model drug doxorubicin (DOX) was coupled to the Fab' fragment of anti-CD20 IgG at its permissive sites through a heterotelechelic PEG linker, generating an antibody fragment-drug conjugate (AFDC). Anti-CD20 IgG was digested and reduced specifically with β-mercaptoethylamine to generate the Fab' fragment with two free mercapto groups in its hinge region. Meanwhile, DOX was conjugated with α-succinimidylsuccinate ω-maleimide polyethylene glycol (NHS-PEG-MAL) to form MAL-PEG-DOX, which was subsequently linked to the free mercapto containing Fab' fragment to form a Fab'-PEG-DOX conjugate. The dual site-specific bioconjugation was achieved through the combination of highly selective reduction of IgG and introduction of heterotelechelic PEG linker. The resulting AFDC provides an utterly homogeneous product, with a definite ratio of one fragment to two drugs. Laser confocal microscopy and cell ELISA revealed that the AFDC could accumulate in the antigen-positive Daudi tumor cell. In addition, the Fab'-PEG-DOX retained appreciable targeting ability and improved antitumor activity, demonstrating an excellent therapeutic effect on the lymphoma mice model for better cure rate and significantly reduced side effects.
-
Side Chain Engineering in Solution-Processable Conjugated Polymers
Mei, Jianguo
2014-01-14
Side chains in conjugated polymers have been primarily utilized as solubilizing groups. However, these side chains have roles that are far beyond. We advocate using side chain engineering to tune a polymer\\'s physical properties, including absorption, emission, energy level, molecular packing, and charge transport. To date, numerous flexible substituents suitable for constructing side chains have been reported. In this Perspective article, we advocate that the side chain engineering approach can advance better designs for next-generation conjugated polymers. © 2013 American Chemical Society.
-
Imaging GABAc Receptors with Ligand-Conjugated Quantum Dots
Directory of Open Access Journals (Sweden)
Ian D. Tomlinson
2007-01-01
Full Text Available We report a methodology for labeling the GABAc receptor on the surface membrane of intact cells. This work builds upon our earlier work with serotonin-conjugated quantum dots and our studies with PEGylated quantum dots to reduce nonspecific binding. In the current approach, a PEGylated derivative of muscimol was synthesized and attached via an amide linkage to quantum dots coated in an amphiphilic polymer derivative of a modified polyacrylamide. These conjugates were used to image GABAC receptors heterologously expressed in Xenopus laevis oocytes.
-
Conjugate Gradient like methods and their application to fixed source neutron diffusion problems
International Nuclear Information System (INIS)
Suetomi, Eiichi; Sekimoto, Hiroshi
1989-01-01
This paper presents a number of fast iterative methods for solving systems of linear equations appearing in fixed source problems for neutron diffusion. We employed the conjugate gradient and conjugate residual methods. In order to accelerate the conjugate residual method, we proposed the conjugate residual squared method by transforming the residual polynomial of the conjugate residual method. Since the convergence of these methods depends on the spectrum of coefficient matrix, we employed the incomplete Choleski (IC) factorization and the modified IC (MIC) factorization as preconditioners. These methods were applied to some neutron diffusion problems and compared with the successive overrelaxation (SOR) method. The results of these numerical experiments showed superior convergence characteristics of the conjugate gradient like method with MIC factorization to the SOR method, especially for a problem involving void region. The CPU time of the MICCG, MICCR and MICCRS methods showed no great difference. In order to vectorize the conjugate gradient like methods based on (M)IC factorization, the hyperplane method was used and implemented on the vector computers, the HITAC S-820/80 and ETA10-E (one processor mode). Significant decrease of the CPU times was observed on the S-820/80. Since the scaled conjugate gradient (SCG) method can be vectorized with no manipulation, it was also compared with the above methods. It turned out the SCG method was the fastest with respect to the CPU times on the ETA10-E. These results suggest that one should implement suitable algorithm for different vector computers. (author)
-
Antioxidative and antiradical properties of plant phenolics.
Sroka, Zbigniew
2005-01-01
The plant phenolic compounds such as flavonoids, tannins and phenolic acids appeared to be strong antiradical and antioxidant compounds. The number of hydroxy groups and the presence of a 2,3-double bond and orthodiphenolic structure enhance antiradical and antioxidative activity of flavonoids. The glycosylation, blocking the 3-OH group in C-ring, lack of a hydroxy group or the presence of only a methoxy group in B-ring have a decreasing effect on antiradical or antioxidative activity of these compounds. Tannins show strong antioxidative properties. Some tannins in red wine or gallate esters were proved to have antioxidative effect in vivo. The number of hydroxy groups connected with the aromatic ring, in ortho or para position relative to each other, enhance antioxidative and antiradical activity of phenolic acids. The substitution of a methoxy group in ortho position to the OH in monophenols seems to favour the antioxidative activity of the former.
-
Chemical and Sensory Quality Preservation in Coated Almonds with the Addition of Antioxidants.
Larrauri, Mariana; Demaría, María Gimena; Ryan, Liliana C; Asensio, Claudia M; Grosso, Nelson R; Nepote, Valeria
2016-01-01
Almonds provide many benefits such as preventing heart disease due to their high content of oleic fatty acid-rich oil and other important nutrients. However, they are susceptible to oxidation reactions causing rancidity during storage. The objective of this work was to evaluate the chemical and sensory quality preservation of almonds coated with carboxymethyl cellulose and with the addition of natural and synthetic antioxidants during storage. Four samples were prepared: almonds without coating (C), almonds coated with carboxymethyl cellulose (CMC), almonds coated with CMC supplemented with peanut skins extract (E), and almonds coated with CMC and supplemented with butylhydroxytoluene (BHT). Proximate composition and fatty acid profile were determined on raw almonds. Almond samples (C, CMC, E and BHT) were stored at 40 °C for 126 d. Lipid oxidation indicators: peroxide value (PV), conjugated dienes (CD), volatile compounds (hexanal and nonanal), and sensory attributes were determined for the stored samples. Samples showed small but significant increases in PV, CD, hexanal and nonanal contents, and intensity ratings of negative sensory attributes (oxidized and cardboard). C had the highest tendency to deterioration during storage. At the end of storage (126 d), C had the highest PV (3.90 meqO2 /kg), and BHT had the lowest PV (2.00 meqO2 /kg). CMC and E samples had similar intermediate PV values (2.69 and 2.57 meqO2 /kg, respectively). CMC coating and the addition of natural (peanut skin extract) and synthetic (BHT) antioxidants provide protection to the roasted almond product. © 2015 Institute of Food Technologists®
-
Information properties of a hologram of mutually conjugate waves
International Nuclear Information System (INIS)
Rubanov, A.S.; Serebryakova, L.M.
1995-01-01
A theoretical study of information properties of a correlation response to a fragment of an image of a thin referenceless hologram of mutually conjugate waves that is recorded with a phase-conjugating (PC) mirror is reported. It is shown that this hologram reconstructs a full image in reflected light and can be used as an associative storage device and as a selective PC mirror. 7 refs., 1 fig
-
Plants as natural antioxidants for meat products
Tomović, V.; Jokanović, M.; Šojić, B.; Škaljac, S.; Ivić, M.
2017-09-01
The meat industry is demanding antioxidants from natural sources to replace synthetic antioxidants because of the negative health consequences or beliefs regarding some synthetic ones. Plants materials provide good alternatives. Spices and herbs, generally used for their flavouring characteristics, can be added to meat products in various forms: whole, ground, or as isolates from their extracts. These natural antioxidants contain some active compounds, which exert antioxidative potential in meat products. This antioxidant activity is most often due to phenolic acids, phenolic diterpenes, flavonoids and volatile oils. Each of these compounds often has strong H-donating activity, thus making them extremely effective antioxidants; some compounds can chelate metals and donate H to oxygen radicals, thus slowing oxidation via two mechanisms. Numerous studies have demonstrated the efficacy of natural antioxidants when used in meat products. Based on this literature review, it can be concluded that natural antioxidants are added to fresh and processed meat and meat products to delay, retard, or prevent lipid oxidation, retard development of off-flavours (rancidity), improve colour stability, improve microbiological quality and extend shelf-life, without any damage to the sensory or nutritional properties.
-
Antioxidant activity of Arbutus unedo leaves.
Pabuçcuoğlu, A; Kivçak, B; Baş, M; Mert, T
2003-09-01
The ethanol and methanol extracts of Arbutus unedo leaves were screened for antioxidant activity. The antioxidant activity was determined by an improved assay based on the decolorization of the radical monocation of [2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] (ABTS). The ethanol and methanol extract of A. unedo leaves displayed potent antioxidant activity.
-
Development of Tat-Conjugated Dendrimer for Transdermal DNA Vaccine Delivery.
Bahadoran, Azadeh; Moeini, Hassan; Bejo, Mohd Hair; Hussein, Mohd Zobir; Omar, Abdul Rahman
In order to enhance cellular uptake and to facilitate transdermal delivery of DNA vaccine, polyamidoamine (PAMAM) dendrimers conjugated with HIV transactivator of transcription (TAT) was developed. First, the plasmid DNA (pIRES-H5/GFP) nanoparticle was formulated using PAMAM dendrimer and TAT peptide and then characterized for surface charge, particle size, DNA encapsulation and protection of the pIRES-H5/GFP DNA plasmid to enzymatic digestion. Subsequently, the potency of the TAT-conjugated dendrimer for gene delivery was evaluated through in vitro transfection into Vero cells followed by gene expression analysis including western blotting, fluorescent microscopy and PCR. The effect of the TAT peptide on cellular uptake of DNA vaccine was studied by qRT-PCR and flow cytometry. Finally, the ability of TAT-conjugated PAMAM dendrimer for transdermal delivery of the DNA plasmid was assessed through artificial membranes followed by qRT-PCR and flow cytometry. TAT-conjugated PAMAM dendrimer showed the ability to form a compact and nanometre-sized polyplexes with the plasmid DNA, having the size range of 105 to 115 nm and a positive charge of +42 to +45 mV over the N/P ratio of 6:1(+/-). In vitro transfection analysis into Vero cells confirms the high potency of TAT-conjugated PAMAM dendrimer to enhance the cellular uptake of DNA vaccine. The permeability value assay through artificial membranes reveals that TAT-conjugated PAMAM has more capacity for transdermal delivery of the DNA compared to unmodified PAMAM dendrimer (Pdendrimer is a promising non-viral vector for transdermal use.This article is open to POST-PUBLICATION REVIEW. Registered readers (see "For Readers") may comment by clicking on ABSTRACT on the issue's contents page.
-
Gamma radiation effects on peanut skin antioxidants
International Nuclear Information System (INIS)
Camargo, Adriano Costa de; Canniatti-Brazaca, Solange Guidolin; Vieira, Thais Maria Ferreira de Souza; Regitano-d'Arce, Marisa Aparecida Bismara; Calori-Domingues, Maria Antonia
2011-01-01
Peanut skin, which is removed in the peanut blanching process, is rich in bioactive compounds with antioxidant properties. The viability of using natural sources of antioxidants to replace synthetic antioxidants was assessed. The aims of this study were to measure bioactive compounds in peanut skins and evaluate the effect of gamma radiation on their antioxidant activity. Peanut skin samples were treated with 0.0, 5.0, 7.5, or 10.0 kGy gamma rays at a dose rate of 7.5 kGy/h using a 60 Co source. Total phenolics, condensed tannins, total flavonoids, and antioxidant activity were evaluated. Extracts obtained from the peanut skins were added to refined-bleached deodorized (RBD) soybean oil that was free from synthetic antioxidants. The oxidative stability of the oil samples was determined using the Rancimat method and compared to a control and synthetic antioxidants (100 mg/kg BHT and 200 mg/kg TBHQ). Gamma radiation changed total phenolic content, total condensed tannins, total flavonoid content, and the antioxidant activity. Ethanolic extracts, gamma irradiated or not, presented increasing induction period (h), measured by the Rancimat method, when compared with the control. Antioxidant activity of the peanut skins was higher than BHT but lower than THBQ. The present study confirmed that gamma radiation did not affect the peanut skin extracts' antioxidative level when added to soybean oil. The induction period of the control soybean oil was 5.7 h, while soybean oil with added ethanolic peanut skin extract had an induction period of 7.2 h, on average. (author)
-
Gamma radiation effects on peanut skin antioxidants
Energy Technology Data Exchange (ETDEWEB)
Camargo, Adriano Costa de [Centro de Energia Nuclear na Agricultura (CENA/USP), Piracicaba, SP (Brazil); Canniatti-Brazaca, Solange Guidolin; Vieira, Thais Maria Ferreira de Souza; Regitano-d' Arce, Marisa Aparecida Bismara; Calori-Domingues, Maria Antonia, E-mail: sgcbraza@usp.b, E-mail: tvieira@esalq.usp.b, E-mail: mabra@esalq.usp.b, E-mail: macdomin@esalq.usp.b [Escola Superior de Agricultura Luiz de Queiroz (ESALQ/USP), Piracicaba, SP (Brazil). Dept. de Agroindustria, Alimentos e Nutricao
2011-07-01
Peanut skin, which is removed in the peanut blanching process, is rich in bioactive compounds with antioxidant properties. The viability of using natural sources of antioxidants to replace synthetic antioxidants was assessed. The aims of this study were to measure bioactive compounds in peanut skins and evaluate the effect of gamma radiation on their antioxidant activity. Peanut skin samples were treated with 0.0, 5.0, 7.5, or 10.0 kGy gamma rays at a dose rate of 7.5 kGy/h using a {sup 60}Co source. Total phenolics, condensed tannins, total flavonoids, and antioxidant activity were evaluated. Extracts obtained from the peanut skins were added to refined-bleached deodorized (RBD) soybean oil that was free from synthetic antioxidants. The oxidative stability of the oil samples was determined using the Rancimat method and compared to a control and synthetic antioxidants (100 mg/kg BHT and 200 mg/kg TBHQ). Gamma radiation changed total phenolic content, total condensed tannins, total flavonoid content, and the antioxidant activity. Ethanolic extracts, gamma irradiated or not, presented increasing induction period (h), measured by the Rancimat method, when compared with the control. Antioxidant activity of the peanut skins was higher than BHT but lower than THBQ. The present study confirmed that gamma radiation did not affect the peanut skin extracts' antioxidative level when added to soybean oil. The induction period of the control soybean oil was 5.7 h, while soybean oil with added ethanolic peanut skin extract had an induction period of 7.2 h, on average. (author)
-
Antioxidants: Characterization, natural sources, extraction and analysis
OROIAN, MIRCEA; Escriche Roberto, Mª Isabel
2015-01-01
[EN] Recently many review papers regarding antioxidants fromdifferent sources and different extraction and quantification procedures have been published. However none of them has all the information regarding antioxidants (chemistry, sources, extraction and quantification). This article tries to take a different perspective on antioxidants for the new researcher involved in this field. Antioxidants from fruit, vegetables and beverages play an important role in human health, fo...
-
Rosen, Christian B.; Kodal, Anne L. B.; Nielsen, Jesper S.; Schaffert, David H.; Scavenius, Carsten; Okholm, Anders H.; Voigt, Niels V.; Enghild, Jan J.; Kjems, Jørgen; Tørring, Thomas; Gothelf, Kurt V.
2014-09-01
DNA-protein conjugates are important in bioanalytical chemistry, molecular diagnostics and bionanotechnology, as the DNA provides a unique handle to identify, functionalize or otherwise manipulate proteins. To maintain protein activity, conjugation of a single DNA handle to a specific location on the protein is often needed. However, preparing such high-quality site-specific conjugates often requires genetically engineered proteins, which is a laborious and technically challenging approach. Here we demonstrate a simpler method to create site-selective DNA-protein conjugates. Using a guiding DNA strand modified with a metal-binding functionality, we directed a second DNA strand to the vicinity of a metal-binding site of His6-tagged or wild-type metal-binding proteins, such as serotransferrin, where it subsequently reacted with lysine residues at that site. This method, DNA-templated protein conjugation, facilitates the production of site-selective protein conjugates, and also conjugation to IgG1 antibodies via a histidine cluster in the constant domain.
-
International Nuclear Information System (INIS)
Khoza, Phindile; Antunes, Edith; Chen, Ji-Yao; Nyokong, Tebello
2013-01-01
This work reports on the synthesis of zinc tetraaminophthalocyanine (ZnTAPc) functionalized with folic acid (FA), forming ZnTAPcFA. The conjugate between FA and ZnTAPc was soluble in water whereas ZnTAPc alone is not. The structure of ZnTAPcFA conjugate was elucidated by 1 H NMR, MALDI-TOF mass and FTIR spectra. Photophysical and photochemical studies of ZnTAPcFA were conducted in DMSO. The increase in fluorescence quantum yield of the conjugate was accompanied by a decrease in the triplet and singlet oxygen quantum yields. The changes in triplet quantum and singlet oxygen quantum yields were marginal when ZnTAPc was simply mixed with FA without a chemical bond. - Highlights: ► A conjugate between folic acid and a zinc tetraaminophthalocyanine was formed. ► The conjugate is water soluble even though the phthalocyanine alone is not. ► The fluorescence quantum yield of the conjugate was enhanced compared to the phthalocyanine alone. ► Triplet quantum yields decreased for the conjugate
-
Zhang, Shuwen; Gong, Yuansheng; Khanal, Som; Lu, Yanjie; Lucey, John A
2017-11-01
Covalent attachment of polysaccharides to proteins (conjugation) via the Maillard reaction has been extensively studied. Conjugation can lead to a significant improvement in protein functionality (e.g., solubility, emulsification, and heat stability). Caseins have previously been successfully conjugated with maltodextrin (Md), but the effect on the detailed acid gelation properties has not been examined. We studied the effect of conjugating sodium caseinate (NaCN) with 3 different sized Md samples via the Maillard reaction in aqueous solutions. The Md samples had dextrose equivalents of 4 to 7, 9 to 12, and 20 to 23 for Md40, Md100, and Md200, respectively. The conjugation reaction was performed in mixtures with 5% NaCN and 5% Md, which were heated at 90°C for 10 h. The degree of conjugation was estimated from the reduction in free amino groups as well as color changes. Sodium dodecyl sulfate-PAGE analysis was performed to confirm conjugation by employing staining of both protein and carbohydrate bands. The molar mass of samples was determined by size-exclusion chromatography coupled with multi-angle laser light scattering. After the conjugation reaction, samples were then gelled by the addition of 0.63% (wt/vol) glucono-δ-lactone at 30°C, such that samples reached pH 4.6 after about 13 h. The rheological properties of samples during acidification was monitored by small-strain dynamic oscillatory rheology. The microstructure of acid gels at pH 4.6 was examined by fluorescence microscopy. Conjugation resulted in a loss of 10.8, 8.8, and 11.9% of the available amino groups in the protein for the NaCN-Md40 conjugates (C40), NaCN-Md100 conjugates (C100), and NaCN-Md100 conjugates (C200), respectively. With a decrease in the size of the type of Md, an increase occurred in the molar mass of the resultant conjugate. The weight average molar masses of NaCN-Md samples were 340, 368, and 425 kDa for the conjugates C40, C100, and C200, respectively. Addition of Md to Na
-
Levine, Paul M.; Lee, Eugine; Greenfield, Alex; Bonneau, Richard; Logan, Susan K.; Garabedian, Michael J.; Kirshenbaum, Kent
2013-01-01
Sustained treatment of prostate cancer with Androgen Receptor (AR) antagonists can evoke drug resistance, leading to castrate-resistant disease. Elevated activity of the AR is often associated with this highly aggressive disease state. Therefore, new therapeutic regimens that target and modulate AR activity could prove beneficial. We previously introduced a versatile chemical platform to generate competitive and non-competitive multivalent peptoid oligomer conjugates that modulate AR activity. In particular, we identified a linear and a cyclic divalent ethisterone conjugate that exhibit potent anti-proliferative properties in LNCaP-abl cells, a model of castrate-resistant prostate cancer. Here, we characterize the mechanism of action of these compounds utilizing confocal microscopy, time-resolved fluorescence resonance energy transfer, chromatin immunoprecipitation, flow cytometry, and microarray analysis. The linear conjugate competitively blocks AR action by inhibiting DNA binding. In addition, the linear conjugate does not promote AR nuclear localization or co-activator binding. In contrast, the cyclic conjugate promotes AR nuclear localization and induces cell-cycle arrest, despite its inability to compete against endogenous ligand for binding to AR in vitro. Genome-wide expression analysis reveals that gene transcripts are differentially affected by treatment with the linear or cyclic conjugate. Although the divalent ethisterone conjugates share extensive chemical similarities, we illustrate that they can antagonize the AR via distinct mechanisms of action, establishing new therapeutic strategies for potential applications in AR pharmacology. PMID:22871957
-
Excitation energy deactivation funnel in 3-substituted BODIPY-porphyrin conjugate
International Nuclear Information System (INIS)
Nguyen, Nguyen Tran; Verbelen, Bram; Leen, Volker; Waelkens, Etienne; Dehaen, Wim; Kruk, Mikalai
2016-01-01
BODIPYs absorb in the visible region which is complementary to that of porphyrins and therefore can be suggested as promising antenna groups to improve the light-harvesting potential of porphyrins. A boron-dipyrromethene dye was combined at the 3-position with a Zn-porphyrin to afford a conjugate. The fluorescence of the conjugate was found to originate from the BODIPY moiety independently of the excitation wavelength due to an unique set of energy transfer rates between the BODIPY and Zn-porphyrin moieties. The fluorescence intensity was shown to be tunable over a wide range using the solvent properties. This feature makes the studied BODIPY-porphyrin conjugate a promising compound for the design of new photochromic devices.
-
Bifunctional rhodium intercalator conjugates as mismatch-directing DNA alkylating agents.
Schatzschneider, Ulrich; Barton, Jacqueline K
2004-07-21
A conjugate of a DNA mismatch-specific rhodium intercalator, containing the bulky chrysenediimine ligand, and an aniline mustard has been prepared, and targeting of mismatches in DNA by this conjugate has been examined. The preferential alkylation of mismatched over fully matched DNA is found by a mobility shift assay at concentrations where untethered organic mustards show little reaction. The binding site of the Rh intercalator was determined by DNA photocleavage, and the position of covalent modification was established on the basis of the enhanced depurination associated with N-alkylation. The site-selective alkylation at mismatched DNA renders these conjugates useful tools for the covalent tagging of DNA base pair mismatches and new chemotherapeutic design.
-
Aptamer-conjugated dendrimer-modified quantum dots for glioblastoma cells imaging
International Nuclear Information System (INIS)
Li Zhiming; Huang Peng; He Rong; Bao Chenchen; Cui Daxiang; Zhang Xiaomin; Ren Qiushi
2009-01-01
Targeted quantum dots have shown potential as a platform for development of cancer imaging. Aptamers have recently been demonstrated as ideal candidates for molecular targeting applications. In present work, polyamidoamine dendrimers were used to modify surface of quantum dots and improve their solubility in water solution. Then, dendrimer-modified quantum dots were conjugated with DNA aptamer, GBI-10, can recognize the extracellular matrix protein tenascin-C on the surface of human glioblastoma cells. The dendrimer-modified quantum dots exhibit water-soluble, high quantum yield, and good biocompatibility. Aptamer-conjugated quantum dots can specifically target U251 human glioblastoma cells. High-performance aptamer-conjugated dendrimers modified quantum dot-based nanoprobes have great potential in application such as cancer imaging.
-
Excitation energy deactivation funnel in 3-substituted BODIPY-porphyrin conjugate
Energy Technology Data Exchange (ETDEWEB)
Nguyen, Nguyen Tran [Chemistry Department, University of Education, The University of DaNang, Ton Duc Thang 459, Da Nang (Viet Nam); Molecular Design and Synthesis, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, B-3001 Leuven (Belgium); Verbelen, Bram; Leen, Volker [Molecular Design and Synthesis, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, B-3001 Leuven (Belgium); Waelkens, Etienne [Department of Cellular and Molecular Medicine, KU Leuven, Herestraat 49, Box 901, 3000 Leuven (Belgium); Dehaen, Wim, E-mail: wim.dehaen@kuleuven.be [Molecular Design and Synthesis, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, B-3001 Leuven (Belgium); Kruk, Mikalai, E-mail: m.kruk@belstu.by [Belarusian State Technological University, Physics Department, Sverdlov Str., 13a, Minsk 220006 (Belarus)
2016-11-15
BODIPYs absorb in the visible region which is complementary to that of porphyrins and therefore can be suggested as promising antenna groups to improve the light-harvesting potential of porphyrins. A boron-dipyrromethene dye was combined at the 3-position with a Zn-porphyrin to afford a conjugate. The fluorescence of the conjugate was found to originate from the BODIPY moiety independently of the excitation wavelength due to an unique set of energy transfer rates between the BODIPY and Zn-porphyrin moieties. The fluorescence intensity was shown to be tunable over a wide range using the solvent properties. This feature makes the studied BODIPY-porphyrin conjugate a promising compound for the design of new photochromic devices.
-
Jain, S K; Gill, M S; Pawar, H S; Suresh, Sarasija
2014-09-01
Curcumin-diclofenac conjugate as been synthesized by esterification of phenolic group of curcumin with the acid moiety of diclofenac, and characterized by mass spectrometry, NMR, FTIR, DSC, thermogravimetric analysis and X-ray diffraction analysis. The relative solubility of curcumin-diclofenac conjugate, curcumin and diclofenac; stability of curcumin-diclofenac conjugate in intestinal extract; permeability study of curcumin-diclofenac conjugate using the everted rat intestinal sac method; stability of curcumin-diclofenac conjugate in gastrointestinal fluids and in vitro efficacy have been evaluated. In vivo bioavailability of curcumin-diclofenac conjugate and curcumin in Sprague-Dawley rats, and antiarthritic activity of curcumin-diclofenac conjugate, curcumin and diclofenac in modified streptococcal cell wall-induced arthritis model in Balb/c mice to mimic rheumatoid arthritis in humans have also been studied. In all of the above studies, curcumin-diclofenac conjugate exhibited enhanced stability as compared to curcumin; its activity was twice that of diclofenac in inhibiting thermal protein denaturation taken as a measure of in vitro antiinflammatory activity; it enhanced the bioavailability of curcumin by more than five folds, and significantly (Parthritis in streptococcal cell wall-induced arthritis model as compared to both diclofenac and curcumin.
-
Photo-excitation of carotenoids causes cytotoxicity via singlet oxygen production
International Nuclear Information System (INIS)
Yoshii, Hiroshi; Yoshii, Yukie; Asai, Tatsuya; Furukawa, Takako; Takaichi, Shinichi; Fujibayashi, Yasuhisa
2012-01-01
Highlights: ► Some photo-excited carotenoids have photosensitizing ability. ► They are able to produce ROS. ► Photo-excited fucoxanthin can produce singlet oxygen through energy transfer. -- Abstract: Carotenoids, natural pigments widely distributed in algae and plants, have a conjugated double bond system. Their excitation energies are correlated with conjugation length. We hypothesized that carotenoids whose energy states are above the singlet excited state of oxygen (singlet oxygen) would possess photosensitizing properties. Here, we demonstrated that human skin melanoma (A375) cells are damaged through the photo-excitation of several carotenoids (neoxanthin, fucoxanthin and siphonaxanthin). In contrast, photo-excitation of carotenoids that possess energy states below that of singlet oxygen, such as β-carotene, lutein, loroxanthin and violaxanthin, did not enhance cell death. Production of reactive oxygen species (ROS) by photo-excited fucoxanthin or neoxanthin was confirmed using a reporter assay for ROS production with HeLa Hyper cells, which express a fluorescent indicator protein for intracellular ROS. Fucoxanthin and neoxanthin also showed high cellular penetration and retention. Electron spin resonance spectra using 2,2,6,6-tetramethil-4-piperidone as a singlet oxygen trapping agent demonstrated that singlet oxygen was produced via energy transfer from photo-excited fucoxanthin to oxygen molecules. These results suggest that carotenoids such as fucoxanthin, which are capable of singlet oxygen production through photo-excitation and show good penetration and retention in target cells, are useful as photosensitizers in photodynamic therapy for skin disease.
-
99Tcm labelling and in vitro binding of dextran-somatostatin conjugate
International Nuclear Information System (INIS)
Cui Haiping; China Inst. of Atomic Energy, Beijing; Zhai Shizhen; Du Jin; Beijing Univ., Beijing
2006-01-01
Natural somatostatin and dextran-20 are used to synthesis somatostatin-dextran (SMS-Dx 20 ). The in vitro somatostatin receptor competition binding study of somatostatin-dextran is carried out by using rate brain cortex membranes (express somatostatin receptor type 2) and 125 I-Tyr 3 -Octreotide as a radioligand. The somatostatin-dextran conjugate is then labelled with 99 Tc m using SnCl 2 as reduce agent and tested for its in vitro binding properties. The somatostatin-dextran conjugate shows high somatostatin receptor binding affinity, i.e. in the same IC 50 value as the reference ligand Octreotide (IC 50 ∼5.95 nmol/L). The labelling efficiency is more than 85%. The specific binding of 99 Tc m labeled somatostatin-dextran conjugate is 25%-40%. The somatostatin-dextran conjugate is worthy of further investigation for 99 Tc m radiolabeling with diagnostic possibilities for somatostatin receptor positive tumors. (authors)
-
Energy Technology Data Exchange (ETDEWEB)
Golkar, Nasim; Samani, Soliman Mohammadi; Tamaddon, Ali Mohammad, E-mail: amtamadon@gmail.com [Shiraz University of Medical Sciences, Department of Pharmaceutics, School of Pharmacy (Iran, Islamic Republic of)
2016-05-15
Aimed to prepare an enhanced gene delivery system with low cytotoxicity and high transfection efficiency, various cholesterol-conjugated derivates of low generation polyamidoamine (PAMAM) dendrimers were prepared. The conjugates were characterized by TNBS assay, FTIR, and {sup 1}H-NMR spectroscopy. Self-assembly of the dendrimer conjugates (G1-Chol, G2-Chol, and G3-Chol) was investigated by pyrene assay. Following formation of the complexes between enhanced green fluorescence protein plasmid and the dendrimer conjugates at various N (primary amine)/P (phosphate) mole ratios, plasmid condensation, biologic stability, cytotoxicity, and protein expression were investigated. The conjugates self-assembled into micellar dispersions with the critical micelle concentration values (<50 µg/ml) depending on the dendrimer generation and cholesterol/amine mole ratio. Cholesterol conjugation resulted in higher resistance of the condensed plasmid DNA in a competition assay with heparin sulfate. Also, the transfection efficiency was determined higher for the cholesterol conjugates than unmodified dendrimers in HepG2 cells, showing the highest for G2-Chol at 40 % degree of cholesterol modification (G2-Chol{sub 40 %}) among various dendrimer generations. Interestingly, such conjugate showed a complete protection of plasmid against serum nucleases. Our results confirmed that the cholesterol conjugation to PAMAM dendrimers of low generations bearing little cytotoxicity improves their several physicochemical and biological characteristics required for an enhanced delivery of plasmid DNA into cells.
-
Antioxidants Potential of the Filamentous Fungi (Mucor circinelloides).
Hameed, Ahsan; Hussain, Syed Ammar; Yang, Junhuan; Ijaz, Muhammad Umair; Liu, Qing; Suleria, Hafiz Ansar Rasul; Song, Yuanda
2017-10-07
Three important strains of Mucor circinelloides grown in complete and minimal media for specified period (72 h, 120 h and 168 h) under submerged fermentation conditions were investigated for their potential antioxidants/secondary metabolite production. All mycelial extracts demonstrated effective antioxidant activities in terms of β-carotene/linoleic acid bleaching, radical scavenging, reduction of metal ions and chelating abilities against ferrous ions. Different extraction methods and solvent systems affected the recovery yield and antioxidant activities of the extracts significantly ( p ≤ 0.05). Ethanolic extracts were found to be rich source of antioxidant components and subsequently more effective in antioxidant properties. Fermentation period and media used also significantly affected ( p ≤ 0.05) the antioxidant production and the resulting antioxidant properties. The (ethanolic) extracts of all the strains from late exponential growth phase (120 h) showed highest antioxidant production with topmost reducing, chelating and radical scavenging capabilities. Strain MC277.49 was found to be the highest producer of antioxidants followed by MC108.16 and WJ11. Phenolic compounds were detected significantly in higher ( p ≤ 0.05) amount succeeded by the condensed tannins and flavonoids. Total phenol content of each extract was attributed to overall antioxidant capacity. Submerged fermentation with nutritional stress conditions were found to be excellent way of producing surplus amount of natural antioxidants/secondary metabolites with their vast potential commercial application in food and pharmaceutical industries.
-
McDowell, Arlene; Thompson, Scott; Stark, Mirjam; Ou, Zong-Quan; Gould, Kevin S
2011-12-01
There is considerable interest in antioxidant dietary components that can be protective against degenerative diseases in humans. Puha (Sonchus oleraceus L.) is a rich source of polyphenols, and exhibits strong antioxidant activity as measured by the 2,2-diphenylpicrylhydrazyl (DPPH) assay. However, the potential of puha to protect against degenerative diseases requires that low molecular weight antioxidants (LMWA) are absorbed by, and active in, human cells. The cellular antioxidant activity (CAA) assay was used to investigate the antioxidant activity of puha leaf extracts. Preparation methods of freezing and freeze-drying reduced the total polyphenolic content compared with fresh puha, but did not affect the LMWA potential as determined by the DPPH assay. The IC(50) values were 0.012 ± 0.003 mg/mL and 0.010 ± 0.005 mg/mL for freeze-dried and fresh puha leaves, respectively. Using the CAA assay, it was shown that LMWAs from foliar extracts of puha were effectively absorbed into HepG2 cells, and exerted antioxidant activity at levels comparable to those of extracts from blueberry fruits, the much-touted antioxidant superfood. Methylene blue staining of HepG2 cells indicated that puha extracts were not cytotoxic at concentrations below 100 mg DW/mL. The data indicate the potential of puha as a nutraceutical supplement for human health. Copyright © 2011 John Wiley & Sons, Ltd.
-
Popović, Boris M; Stajner, Dubravka; Slavko, Kevrešan; Sandra, Bijelić
2012-09-15
Ethanol extracts (80% in water) of 10 cornelian cherry (Cornus mas L.) genotypes were studied for antioxidant properties, using methods including DPPH(), ()NO, O(2)(-) and ()OH antiradical powers, FRAP, total phenolic and anthocyanin content (TPC and ACC) and also one relatively new, permanganate method (permanganate reducing antioxidant capacity-PRAC). Lipid peroxidation (LP) was also determined as an indicator of oxidative stress. The data from different procedures were compared and analysed by multivariate techniques (correlation matrix calculation and principal component analysis (PCA)). Significant positive correlations were obtained between TPC, ACC and DPPH(), ()NO, O(2)(-), and ()OH antiradical powers, and also between PRAC and TPC, ACC and FRAP. PCA found two major clusters of cornelian cherry, based on antiradical power, FRAP and PRAC and also on chemical composition. Chemometric evaluation showed close interdependence between PRAC method and FRAP and ACC. There was a huge variation between C. mas genotypes in terms of antioxidant activity. Copyright © 2012 Elsevier Ltd. All rights reserved.
-
Conjugal plasmid transfer (pAM beta 1) in Lactobacillus plantarum.
Shrago, A W; Chassy, B M; Dobrogosz, W J
1986-01-01
The streptococcal plasmid pAM beta 1 (erythromycin resistance) was transferred via conjugation from Streptococcus faecalis to Lactobacillus plantarum and was transferred among L. plantarum strains. Streptococcus sanguis Challis was transformed with pAM beta 1 isolated from these transconjugants, and transformants harboring intact pAM beta 1 could conjugate the plasmid back to L. plantarum.
-
Zargoosh, Kiomars; Ghayeb, Yousef; Azmoon, Behnaz; Qandalee, Mohammad
2013-08-01
A simple and fast procedure is described for evaluating the antioxidant activity of hydrophilic and hydrophobic compounds by using the peroxyoxalate-chemiluminescence (PO-CL) reaction of Bis(2,4,6-trichlorophenyl) oxalate (TCPO) with hydrogen peroxide in the presence of di(tert-butyl)2-(tert-butylamino)-5-[(E)-2-phenyl-1-ethenyl]3,4-furandicarboxylate as a highly fluorescent fluorophore. The IC50 values of the well-known antioxidants were calculated and the results were expressed as gallic equivalent antioxidant capacity (GEAC). It was found that the proposed method is free of physical quenching and oxidant interference, for this reason, proposed method is able to determine the accurate scavenging activity of the antioxidants to the free radicals. Finally, the proposed method was applied to the evaluation of antioxidant activity of complex real samples such as soybean oil and sunflower oil (as hydrophobic samples) and honey (as hydrophilic sample). To the best of our knowledge, this is the first time that total antioxidant activity can be determined directly in soybean oil, sunflower oil and honey (not in their extracts) using PO-CL reactions.
-
Jain, S. K.; Gill, M. S.; Pawar, H. S.; Suresh, Sarasija
2014-01-01
Curcumin-diclofenac conjugate as been synthesized by esterification of phenolic group of curcumin with the acid moiety of diclofenac, and characterized by mass spectrometry, NMR, FTIR, DSC, thermogravimetric analysis and X-ray diffraction analysis. The relative solubility of curcumin-diclofenac conjugate, curcumin and diclofenac; stability of curcumin-diclofenac conjugate in intestinal extract; permeability study of curcumin-diclofenac conjugate using the everted rat intestinal sac method; st...
-
Ketopantoyl lactone reductase is a conjugated polyketone reductase.
Hata, H; Shimizu, S; Hattori, S; Yamada, H
1989-03-01
Ketopantoyl lactone reductase (EC 1.1.1.168) of Saccharomyces cerevisiae was found to catalyze the reduction of a variety of natural and unnatural conjugated polyketone compounds and quinones, such as isatin, ninhydrin, camphorquinone and beta-naphthoquinone in the presence of NADPH. 5-Bromoisatin is the best substrate for the enzyme (Km = 3.1 mM; Vmax = 650 mumol/min/mg). The enzyme is inhibited by quercetin, and several polyketones. These results suggest that ketopantoyl lactone reductase is a carbonyl reductase which specifically catalyzes the reduction of conjugated polyketones.
-
Conjugate gradient heat bath for ill-conditioned actions.
Ceriotti, Michele; Bussi, Giovanni; Parrinello, Michele
2007-08-01
We present a method for performing sampling from a Boltzmann distribution of an ill-conditioned quadratic action. This method is based on heat-bath thermalization along a set of conjugate directions, generated via a conjugate-gradient procedure. The resulting scheme outperforms local updates for matrices with very high condition number, since it avoids the slowing down of modes with lower eigenvalue, and has some advantages over the global heat-bath approach, compared to which it is more stable and allows for more freedom in devising case-specific optimizations.
-
Electronic states of emodin and its conjugate base
DEFF Research Database (Denmark)
Nguyen, Son Chi; Hansen, Bjarke Knud Vilster; Hoffmann, Søren Vrønning
2008-01-01
The electronic transitions of emodin (1,3,8-trihydroxy-6-methyl-9,10-anthraquinone, E) and its conjugate base (3-oxido-6-methyl-1,8-dihydroxy-9,10-anthraquinone, Ecb) were investigated by UV-Vis linear dichroism (LD) spectroscopy on molecular samples aligned in stretched poly(vinylalcohol). The e......The electronic transitions of emodin (1,3,8-trihydroxy-6-methyl-9,10-anthraquinone, E) and its conjugate base (3-oxido-6-methyl-1,8-dihydroxy-9,10-anthraquinone, Ecb) were investigated by UV-Vis linear dichroism (LD) spectroscopy on molecular samples aligned in stretched poly...
-
Repenko, Tatjana; Kuehne, Alexander J. C.
2015-10-01
Fluorescent biomedical markers of today such as dye-infiltrated colloids, microgels and quantum dots suffer from fast bleaching, lack surface functionality (for targets or pharmaceutical agents) and potentially leach heavy metals in case of quantum dots (e.g. Cd). By contrast, conjugated polymer particles are non-cytotoxic, exhibit reduced bleaching, as the entire particle consists of fluorophore, they are hydrophobic and show high quantum yields. Consequently, conjugated polymer particles represent ideal materials for biological applications and imaging. However currently, conjugated polymer particles for biomedical imaging usually lack near-infrared (NIR) emission and are polydisperse. Fluorescent agents with emission in the NIR spectrum are interesting for biomedical applications due to their low photo-damage towards biological species and the ability of NIR radiation to penetrate deep into biological tissue.. I will present the development and synthesis of new conjugated polymers particles with fluorescence in the NIR spectral region for bio-imaging and clinical diagnosis. The particle synthesis proceeds in a one-step Pd or Ni-catalyzed dispersion polymerization of functional NIR emitters. The resulting monodisperse conjugated polymer particles are obtained as a dispersion in a non-hazardous solvent. Different sizes in the sub-micrometer range with a narrow size distribution can be produced. Furthermore biological recognition motifs can be easily attached to the conjugated polymers via thiol-yne click-chemistry providing specific tumor targeting without quenching of the fluorescence. References [1] Kuehne AJC, Gather MC, Sprakel J., Nature Commun. 2012, 3, 1088. [2] Repenko T, Fokong S, De Laporte L, Go D, Kiessling F, Lammers T, Kuehne AJC.,Chem Commun 2015, accepted.
-
Reductive nanocomplex encapsulation of cRGD-siRNA conjugates for enhanced targeting to cancer cells
Directory of Open Access Journals (Sweden)
Zhou Z
2017-10-01
Full Text Available Zhaoxiu Zhou,* Shuang Liu,* Yanfen Zhang, Xiantao Yang, Yuan Ma, Zhu Guan, Yun Wu, Lihe Zhang, Zhenjun Yang State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, People’s Republic of China *These authors contributed equally to this work Abstract: In this study, through covalent conjugation and lipid material entrapment, a combined modification strategy was established for effective delivery of small interfering RNA (siRNA. Single strands of siRNA targeting to BRAFV600E gene (siMB3 conjugated with cRGD peptide at 3'-terminus or 5'-terminus via cleavable disulfide bond was synthesized and then annealed with corresponding strands to obtain single and bis-cRGD-siRNA conjugates. A cationic lipid material (CLD developed by our laboratory was mixed with the conjugates to generate nanocomplexes; their uniformity and electrical property were revealed by particle size and zeta potential measurement. Compared with CLD/siBraf, CLD/cRGD-siBraf achieved higher cell uptake and more excellent tumor-targeting ability, especially 21 (sense-5′/antisense-3″-cRGD-congjugate nanocomplex. Moreover, they can regulate multiple pathways to varying degree and reduce acidification of endosome. Compared with the gene silencing of different conjugates, single or bis-cRGD-conjugated siRNA showed little differences except 22 (5/5 which cRGD was conjugated at 5'-terminus of antisense strand and sense strand. However bis-cRGD conjugate 21 nanocomplex exhibited better specific target gene silencing at multiple time points. Furthermore, the serum stabilities of the bis-cRGD conjugates were higher than those of the single-cRGD conjugates. In conclusion, all these data suggested that CLD/bis-conjugates, especially CLD/21, can be an effective system for delivery of siRNA to target BRAFV600E gene for therapy of melanoma. Keywords: cRGD-siRNA conjugates, cationic lipids, targeting, silencing, intracellular pathways
-
Tong, Jing
2013-01-07
reached a maximum value of 0.08% ID/g (percent of the injected dose taken up per gram of brain) 4 h postinjection. The entry of SOD1-(cc)-P(EtOx-b-BuOx) to the brain was mediated by a nonsaturable mechanism. Altogether, SOD1-POx conjugates are promising candidates as macromolecular antioxidant therapies for superoxide-associated diseases such as Ang II-induced neurocardiovascular diseases.
-
International Nuclear Information System (INIS)
Hmamouchi, M.
2000-01-01
In the medical field, the oxidation phenomenon is the source of several pathologies (diabetes, cystic fibrosis, cancers,...). The natural oxidants are used as food preserving and skin ageing moderators. Several plant extracts with antioxidant activity were studied, this important antioxidant activity is probably due to their richness of compounds: polyphenols, phenolic acids, tocopherols, carotenoids, flavonoids,... Many techniques for evaluation and reactional mechanism study of the antioxidative activity are used. After selection, extraction, fractionation, activity screening, chemical analyses of molecules contained in the best active extracts, biological properties research of isolated redox pharmacophore, we have : - determined the structure of active products by spectroscopy and chromatography; - studied the antioxidative properties by EPR and spin trapping of the obtained extracts and molecules. The results of this first part of our work consists in evaluating the antioxidative degree of a great number of natural active principles, extracted from moroccan plants and pur obtained products. The second part consists in studying the action mechanisms using the LDL labelling (F. M.)
-
Occurrence of Conjugated Linolenic Acids in Purified Soybean Oil
Kinami, Tomohisa; Horii, Naoto; Narayan, Bhaskar; Arato, Shingo; Hosokawa, Masashi; Miyashita, Kazuo; Negishi, Hironori; Ikuina, Junichi; Noda, Ryuji; Shirasawa, Seiichi
2007-01-01
A high-performance liquid chromatographic (HPLC) method is described for the determination of conjugated linoleic acids (CLA) and conjugated linolenic acids (CLN). Methyl esters prepared from purified lipid fractions of soybean oil were analyzed using an HPLC system equipped with photodiode-array detector to detect peaks having maximum absorption around 233 and 275 nm. These peaks were concentrated by AgNO3-silicic acid column chromatography and reversed-phase HPLC. The structural analysis, o...
-
Ye, Long; Zhang, Shaoqing; Huo, Lijun; Zhang, Maojie; Hou, Jianhui
2014-05-20
As researchers continue to develop new organic materials for solar cells, benzo[1,2-b:4,5-b']dithiophene (BDT)-based polymers have come to the fore. To improve the photovoltaic properties of BDT-based polymers, researchers have developed and applied various strategies leading to the successful molecular design of highly efficient photovoltaic polymers. Novel polymer materials composed of two-dimensional conjugated BDT (2D-conjugated BDT) have boosted the power conversion efficiency of polymer solar cells (PSCs) to levels that exceed 9%. In this Account, we summarize recent progress related to the design and synthesis of 2D-conjugated BDT-based polymers and discuss their applications in highly efficient photovoltaic devices. We introduce the basic considerations for the construction of 2D-conjugated BDT-based polymers and systematic molecular design guidelines. For example, simply modifying an alkoxyl-substituted BDT to form an alkylthienyl-substituted BDT can improve the polymer hole mobilities substantially with little effect on their molecular energy level. Secondly, the addition of a variety of chemical moieties to the polymer can produce a 2D-conjugated BDT unit with more functions. For example, the introduction of a conjugated side chain with electron deficient groups (such as para-alkyl-phenyl, meta-alkoxyl-phenyl, and 2-alkyl-3-fluoro-thienyl) allowed us to modulate the molecular energy levels of 2D-conjugated BDT-based polymers. Through the rational design of BDT analogues such as dithienobenzodithiophene (DTBDT) or the insertion of larger π bridges, we can tune the backbone conformations of these polymers and modulate their photovoltaic properties. We also discuss the influence of 2D-conjugated BDT on polymer morphology and the blends of these polymers with phenyl-C61 (or C71)-butyric acid methyl ester (PCBM). Finally, we summarize the various applications of the 2D-conjugated BDT-based polymers in highly efficient PSC devices. Overall, this Account
-
Thin Films Formed from Conjugated Polymers with Ionic, Water-Soluble Backbones
Voortman, Thomas P; Chiechi, Ryan C
2015-01-01
This paper compares the morphologies of films of conjugated polymers in which the backbone (main chain) and pendant groups are varied between ionic/hydrophilic and aliphatic/hydrophobic. We observe that conjugated polymers in which the pendant groups and backbone are matched, either ionic-ionic or
-
Shofian, Norshahida Mohamad; Hamid, Azizah Abdul; Osman, Azizah; Saari, Nazamid; Anwar, Farooq; Dek, Mohd Sabri Pak; Hairuddin, Muhammad Redzuan
2011-01-01
The effects of freeze-drying on antioxidant compounds and antioxidant activity of five tropical fruits, namely starfruit (Averrhoa carambola L.), mango (Mangifera indica L.), papaya (Carica papaya L.), muskmelon (Cucumis melo L.), and watermelon Citruluss lanatus (Thunb.) were investigated. Significant (p dried fruit samples, except muskmelon. There was no significant (p > 0.05) change, however, observed in the ascorbic acid content of the fresh and freeze-dried fruits. Similarly, freeze-drying did not exert any considerable effect on β-carotene concentration of fruits, except for mango and watermelon, where significantly (p dried fruits. Overall, in comparison to β-carotene and ascorbic acid, a good correlation was established between the result of TPC and antioxidant assays, indicating that phenolics might have been the dominant compounds contributing towards the antioxidant activity of the fruits tested.
-
Two-dimensional charge transport in self-organized, high-mobility conjugated polymers
DEFF Research Database (Denmark)
Sirringhaus, H.; Brown, P.J.; Friend, R.H.
1999-01-01
Self-organization in many solution-processed, semiconducting conjugated polymers results in complex microstructures, in which ordered microcrystalline domains are embedded in an amorphous matrix(I). This has important consequences for electrical properties of these materials: charge transport...... of the ordered microcrystalline domains in the conjugated polymer poly(3-hexylthiophene), P3HT, Self-organization in P3HT results in a lamella structure with two-dimensional conjugated sheets formed by interchain stacking. We find that, depending on processing conditions, the lamellae can adopt two different...... of polymer transistors in logic circuits(5) and active-matrix displays(4,6)....
-
PEG conjugates in clinical development or use as anticancer agents: an overview.
Pasut, Gianfranco; Veronese, Francesco M
2009-11-12
During the almost forty years of PEGylation, several antitumour agents, either proteins, peptides or low molecular weight drugs, have been considered for polymer conjugation but only few entered clinical phase studies. The results from the first clinical trials have shared and improved the knowledge on biodistribution, clearance, mechanism of action and stability of a polymer conjugate in vivo. This has helped to design conjugates with improved features. So far, most of the PEG conjugates comprise of a protein, which in the native form has serious shortcomings that limit the full exploitation of its therapeutic action. The main issues can be short in vivo half-life, instability towards degrading enzymes or immunogenicity. PEGylation proved to be effective in shielding sensitive sites at the protein surface, such as antigenic epitopes and enzymatic degradable sequences, as well as in prolonging the drug half-life by decreasing the kidney clearance. In this review PEG conjugates of proteins or low molecular weight drugs, in clinical development or use as anticancer agents, will be taken into consideration. In the case of PEG-protein derivatives the most represented are depleting enzymes, which act by degrading amino acids essential for cancer cells. Interestingly, PEGylated conjugates have been also considered as adjuvant therapy in many standard anticancer protocols, in this regard the case of PEG-G-CSF and PEG-interferons will be presented.
-
Huang, Richard Y.-C.; O'Neil, Steven R.; Lipovšek, Daša; Chen, Guodong
2018-05-01
Higher-order structure (HOS) characterization of therapeutic protein-drug conjugates for comprehensive assessment of conjugation-induced protein conformational changes is an important consideration in the biopharmaceutical industry to ensure proper behavior of protein therapeutics. In this study, conformational dynamics of a small therapeutic protein, adnectin 1, together with its drug conjugate were characterized by hydrogen/deuterium exchange mass spectrometry (HDX-MS) with different spatial resolutions. Top-down HDX allows detailed assessment of the residue-level deuterium content in the payload conjugation region. HDX-MS dataset revealed the ability of peptide-based payload/linker to retain deuterium in HDX experiments. Combined results from intact, top-down, and bottom-up HDX indicated no significant conformational changes of adnectin 1 upon payload conjugation. [Figure not available: see fulltext.
-
Metal chelate conjugated monoclonal antibodies, wherein the metal is an α emitter
International Nuclear Information System (INIS)
Gansow, O.A.; Strand, M.
1984-01-01
Methods of manufacturing and purifying metal chelate conjugated monoclonal antibodies are described, wherein the chelated metal emits alpha radiation. The conjugates are suited for therapeutic uses being substantially free of nonchelated radiometal. (author)
-
Research on an antioxidant capacity of honeys
Directory of Open Access Journals (Sweden)
Elżbieta Hołderna-Kędzia
2012-12-01
Full Text Available Human organism is exposed to harmful action of free radicals which are produced as well endogenically as egzogenically. The oxidation activity of free radicals can lead to the conversion of systemic biomolecules. As a consequence, there is a threat of, many severe diseases. Antioxidative agents which occur in natural products (also in honey raise a possibility of protection against the harmful action of above mentioned radicals. Polyphenolic compounds - flavonoids, phenolic acids and ascorbic acid - are the most important antioxidative agents. The research of many authors proves that honey, given orally, shows an antioxidative activity. The level of antioxidative agents in serum after the consumption of honey is high and surpasses the antioxidative activity of tea. Dark honeys (honeydew and heather have considerably higher antioxidative activity in comparison to light ones (acacia, lime, polyfloral.
-
Conjugal Pairing in Escherichia Coli
Indian Academy of Sciences (India)
Home; Journals; Resonance – Journal of Science Education; Volume 13; Issue 8. Conjugal Pairing in Escherichia Coli. Joshua Lederberg. Classics Volume 13 Issue 8 August 2008 pp 793-794. Fulltext. Click here to view fulltext PDF. Permanent link: https://www.ias.ac.in/article/fulltext/reso/013/08/0793-0794 ...
-
Antioxidant Capacity of Beetroot: Traditional vs Novel Approaches.
Carrillo, Celia; Rey, Raquel; Hendrickx, Marc; Del Mar Cavia, María; Alonso-Torre, Sara
2017-09-01
Red beetroot has been ranked among the 10 most potent antioxidant vegetables, although only extraction-based methods have been used to evaluate its total antioxidant capacity. Therefore, the present study aims at comparing the traditional extraction-based method with two more recent approaches (QUENCHER -QUick, Easy, New, CHEap and Reproducible- and GAR -global antioxidant response method), in order to establish their suitability in the case of beetroot. Our results indicate that the total antioxidant capacity of beetroot would be underestimated when using extraction-based procedures, since both QUENCHER and GAR methods resulted in a higher total antioxidant capacity. The effect of a thermal treatment on the total antioxidant capacity of beetroot varies among the methods evaluated and our findings suggest different compounds responsible for the total antioxidant capacity detected in each pre-processing method. Remarkably, the present study demonstrates that the traditional extraction-based method seems useful to screen for (changes in) the "bioavailable" antioxidant potential of the root.
-
Antioxidants Potential of the Filamentous Fungi (Mucor circinelloides
Directory of Open Access Journals (Sweden)
Ahsan Hameed
2017-10-01
Full Text Available Three important strains of Mucor circinelloides grown in complete and minimal media for specified period (72 h, 120 h and 168 h under submerged fermentation conditions were investigated for their potential antioxidants/secondary metabolite production. All mycelial extracts demonstrated effective antioxidant activities in terms of β-carotene/linoleic acid bleaching, radical scavenging, reduction of metal ions and chelating abilities against ferrous ions. Different extraction methods and solvent systems affected the recovery yield and antioxidant activities of the extracts significantly (p ≤ 0.05. Ethanolic extracts were found to be rich source of antioxidant components and subsequently more effective in antioxidant properties. Fermentation period and media used also significantly affected (p ≤ 0.05 the antioxidant production and the resulting antioxidant properties. The (ethanolic extracts of all the strains from late exponential growth phase (120 h showed highest antioxidant production with topmost reducing, chelating and radical scavenging capabilities. Strain MC277.49 was found to be the highest producer of antioxidants followed by MC108.16 and WJ11. Phenolic compounds were detected significantly in higher (p ≤ 0.05 amount succeeded by the condensed tannins and flavonoids. Total phenol content of each extract was attributed to overall antioxidant capacity. Submerged fermentation with nutritional stress conditions were found to be excellent way of producing surplus amount of natural antioxidants/secondary metabolites with their vast potential commercial application in food and pharmaceutical industries.
-
Pump induced normal mode splittings in phase conjugation in a Kerr ...
Indian Academy of Sciences (India)
Abstract. Phase conjugation in a Kerr nonlinear waveguide is studied with counter-propagating normally incident pumps and a probe beam at an arbitrary angle of incidence. Detailed numerical results for the specular and phase conjugated reflectivities are obtained with full account of pump depletion. For sufficient ...
-
Stimulated Brillouin scattering continuous wave phase conjugation in step-index fiber optics.
Massey, Steven M; Spring, Justin B; Russell, Timothy H
2008-07-21
Continuous wave (CW) stimulated Brillouin scattering (SBS) phase conjugation in step-index optical fibers was studied experimentally and modeled as a function of fiber length. A phase conjugate fidelity over 80% was measured from SBS in a 40 m fiber using a pinhole technique. Fidelity decreases with fiber length, and a fiber with a numerical aperture (NA) of 0.06 was found to generate good phase conjugation fidelity over longer lengths than a fiber with 0.13 NA. Modeling and experiment support previous work showing the maximum interaction length which yields a high fidelity phase conjugate beam is inversely proportional to the fiber NA(2), but find that fidelity remains high over much longer fiber lengths than previous models calculated. Conditions for SBS beam cleanup in step-index fibers are discussed.
-
Antioxidant activity in cooked and simulated digested eggs.
Remanan, M K; Wu, J
2014-07-25
The avian egg is an excellent source of nutrients consisting of components with beneficial properties but there is limited knowledge on the effect of cooking methods and gastrointestinal digestion on the antioxidant activity of eggs. The present study was focused on the effect of cooking and simulated gastrointestinal digestion on antioxidant activity of eggs using ORAC, ABTS and DPPH assays. The results suggest that fresh egg yolk has higher antioxidant activity than fresh egg white and whole eggs. Cooking reduced whereas simulated gastrointestinal digestion increased the antioxidant activity of eggs. Boiled egg white hydrolysate showed the highest antioxidant activity; a total of 63 peptides were identified, indicative of the formation of novel antioxidant peptides upon simulated gastrointestinal digestion. This study suggests the potential role of eggs as a dietary source of antioxidants.
-
Irradiation of polyethylene in the presence of antioxidants
Jaworska, E.; Kałuska, I.; Strzelczak-Burlińska, G.; Michalik, J.
The radiation induced reactions in LDPE in the presence of phenolic type antioxidants have been studied. It was shown that various antioxidants can influence the polyethylene network formation and the radical yield in different ways. The dependence of network structure on absorbed doses was determined by gel analysis, hot-set test and extraction of antioxidants for samples irradiated with accelerated electrons. It was found that the antioxidants eluated from polyethylene in higher percentage influence polymer crosslinking to a smaller degree. The ESR studies of γ-irradiated blends of polyethylene with antioxidant indicate the presence of alkyl and phenoxyl radicals. The role of antioxidant molecules on radiation induced reactions in polyethylene-antioxidant systems is considered. The correlation between the network structure and the type of additive in polyethylene is also discussed.
-
Antibody conjugate radioimmunotherapy of superficial bladder cancer
International Nuclear Information System (INIS)
Perkins, Alan; Hopper, Melanie; Murray, Andrea; Frier, Malcolm; Bishop, Mike
2002-01-01
The administration of antibody conjugates for cancer therapy is now proving to be of clinical value. We are currently undertaking a programme of clinical studies using the monoclonal antibody C 595 (gG3) which reacts with the MUC1 glycoprotein antigen that is aberrantly expressed in a high proportion of bladder tumours. Radio immuno conjugates of the C 595 antibody have been produced with high radiolabelling efficiency and immuno reactivity using Tc-99 m and In-111 for diagnostic imaging, and disease staging and the cytotoxic radionuclides Cu-67 and Re-188 for therapy of superficial bladder cancer. A Phase I/II therapeutic trail involving the intravesical administration of antibody directly into the bladder has now begun. (author)
-
Liu, Zhihong; Wang, Yutao; Zhang, Na
2012-07-01
During the past decades, polymer-drug conjugates are one of the hottest topics in novel drug development fields. Amphiphilic polymer-drug conjugates in aqueous solution could form micelles or micelle-like nanoassemblies. Compared with polymer-drug conjugates and the micelles into which drugs are physically entrapped, micelles or micelle-like nanoassemblies based on polymer-drug conjugates bring several additional advantages, including increased drug-loading capacity, enhanced intracellular uptake, reduced systemic toxicity, and improved therapeutic efficacy. This review focuses on recent progress achieved in the research field of micelles or micelle-like nanoassemblies based on polymer-drug conjugates. Firstly, properties of polymers, drugs, and linkers which could be used to build polymer-drug conjugate micelles or micelle-like nanoassemblies are summarized. Then, the characterization methods are described. Finally, the drug-targeting mechanisms are discussed. Micelles or micelle-like nanoassemblies based on polymer-drug conjugates as an emerging platform have the potential to achieve medical treatments with enhanced therapeutic effect. The application of micelles or micelle-like nanoassemblies based on polymer-drug conjugates may give new life to old active compounds abandoned due to their low solubility problems. For clinical application, there is a need to further optimize the properties of the polymer, drug, and linker.
-
Directory of Open Access Journals (Sweden)
C. Berzosa
2011-01-01
Full Text Available Antioxidant defences are essential for cellular redox regulation. Since free-radical production may be enhanced by physical activity, herein, we evaluated the effect of acute exercise on total antioxidant status (TAS and the plasma activities of catalase, glutathione reductase, glutathione peroxidase, and superoxide dismutase and its possible relation to oxidative stress resulting from exercise. Healthy untrained male subjects (=34 performed three cycloergometric tests, including maximal and submaximal episodes. Venous blood samples were collected before and immediately after each different exercise. TAS and enzyme activities were assessed by spectrophotometry. An increase of the antioxidant enzyme activities in plasma was detected after both maximal and submaximal exercise periods. Moreover, under our experimental conditions, exercise also led to an augmentation of TAS levels. These findings are consistent with the idea that acute exercise may play a beneficial role because of its ability to increase antioxidant defense mechanisms through a redox sensitive pathway.
-
Obtaining of the antioxidants by supercritical fluid extraction
Directory of Open Access Journals (Sweden)
Babović Nada V.
2011-01-01
Full Text Available One of the important trends in the food industry today is demand for natural antioxidants from plant material. Synthetic antioxidants such as butylated hydroxytoluene (BHT, and butylated hydroxyanisole (BHA are now being replaced by the natural antioxidants because of theirs possible toxicity and as they may act as promoters of carcinogens. The natural antioxidants may show equivalent or higher antioxidant activity than the endogenous or the synthetic antioxidants. Thus, great effort is being devoted to the search for alternative and cheap sources of natural antioxidants, as well as to the development of efficient and selective extraction techniques. The supercritical fluid extraction (SFE with carbon dioxide is considered to be the most suitable method for producing natural antioxidants for the use in food industry. The supercritical extract does not contain residual organic solvents as in conventional extraction processes, which makes these products suitable for use in food, cosmetic and pharmaceutical industry. The recovery of antioxidants from plant sources involves many problematic aspects: choice of an adequate source (in terms of availability, cost, difference in phenolic content with variety and season; selection of the optimal recovery procedure (in terms of yield, simplicity, industrial application, cost; chemical analysis of extracts (for optimization purposes a fast colorimetric method is more preferable than a chromatographic one; evaluation of the antioxidant power (preferably by the different assay methods. The paper presents information about different operational methods for SFE of bioactive compounds from natural sources. It also includes the various reports on the antioxidant activity of the supercritical extracts from Lamiaceae herbs, in comparison with the activity of the synthetic antioxidants and the extracts from Lamiaceae herbs obtained by the conventional methods.
-
Principles of conjugating quantum dots to proteins via carbodiimide chemistry
International Nuclear Information System (INIS)
Song Fayi; Chan, Warren C W
2011-01-01
The covalent coupling of nanomaterials to bio-recognition molecules is a critical intermediate step in using nanomaterials for biology and medicine. Here we investigate the carbodiimide-mediated conjugation of fluorescent quantum dots to different proteins (e.g., immunoglobulin G, bovine serum albumin, and horseradish peroxidase). To enable these studies, we developed a simple method to isolate quantum dot bioconjugates from unconjugated quantum dots. The results show that the reactant concentrations and protein type will impact the overall number of proteins conjugated onto the surfaces of the quantum dots, homogeneity of the protein–quantum dot conjugate population, quantum efficiency, binding avidity, and enzymatic kinetics. We propose general principles that should be followed for the successful coupling of proteins to quantum dots.
-
Energy Technology Data Exchange (ETDEWEB)
Khoza, Phindile; Antunes, Edith [Department of Chemistry, Rhodes University, PO Box 94, Grahamstown (South Africa); Chen, Ji-Yao [State Key Laboratory of Surface Physics and Department of Physics, Fudan University, Shanghai 200433 (China); Nyokong, Tebello, E-mail: t.nyokong@ru.ac.za [Department of Chemistry, Rhodes University, PO Box 94, Grahamstown (South Africa)
2013-02-15
This work reports on the synthesis of zinc tetraaminophthalocyanine (ZnTAPc) functionalized with folic acid (FA), forming ZnTAPcFA. The conjugate between FA and ZnTAPc was soluble in water whereas ZnTAPc alone is not. The structure of ZnTAPcFA conjugate was elucidated by {sup 1}H NMR, MALDI-TOF mass and FTIR spectra. Photophysical and photochemical studies of ZnTAPcFA were conducted in DMSO. The increase in fluorescence quantum yield of the conjugate was accompanied by a decrease in the triplet and singlet oxygen quantum yields. The changes in triplet quantum and singlet oxygen quantum yields were marginal when ZnTAPc was simply mixed with FA without a chemical bond. - Highlights: Black-Right-Pointing-Pointer A conjugate between folic acid and a zinc tetraaminophthalocyanine was formed. Black-Right-Pointing-Pointer The conjugate is water soluble even though the phthalocyanine alone is not. Black-Right-Pointing-Pointer The fluorescence quantum yield of the conjugate was enhanced compared to the phthalocyanine alone. Black-Right-Pointing-Pointer Triplet quantum yields decreased for the conjugate.
-
International Nuclear Information System (INIS)
Jackson, S.M.
1994-08-01
In this study, the term ''conjugate'' refers to faults that occur in two intersecting sets and coordinated kinematically, with each set being distinctive in both orientation and sense of shear (Davis, 1984). Contemporaneous activity along the conjugate faults is defined as occurring within the time frame of the mainshock-aftershock sequence (three weeks for this sequence and generally less than one month in other observed cases). Detailed recordings of microearthquakes from a dense array of temporary analog seismic stations are analyzed. The focal mechanisms and hypocenter spatial and temporal characteristics are combined with geological information to assess the style, geometry, timing, kinematics, and mechanics of conjugate normal faulting. The characteristics of conjugate normal faulting observed in the Devil Canyon sequence are compared to other conjugate normal faulting sequences, and strike-slip and thrust conjugate sequences worldwide
-
Interaction of phenolic antioxidants and hydroxyl radicals
International Nuclear Information System (INIS)
Wang Wenfeng; Luo Jian; Yao Side; Lian Zhirui; Zhang Jiashan; Lin Nianyun
1992-01-01
Based on pulse radiolysis of aqueous solutions of four phenolic antioxidants including green tea polyphenols, quercetin, caffeic acid and sinapic acid the rate constants for reactions of OH and the antioxidants were determined. And green tea polyphenols and quercetin are the strongest antioxidants
-
Interaction of phenolic antioxidants and hydroxyl radicals
International Nuclear Information System (INIS)
Wang, W.F.; Luo, J.; Yao, S.D.; Lian, Z.R.; Zhang, J.S.; Lin, N.Y.
1993-01-01
Based on pulse radiolysis of aqueous solutions of four phenolic antioxidants including green tea polyphenols, quercetin, caffeic acid and sinapic acid the rate constants for reactions of OH and the antioxidants were determined. Green tea polyphenols and quercetin are the strongest antioxidants. (author)
-
Comparative Antioxidant, Antiproliferative and Apoptotic Effects of ...
African Journals Online (AJOL)
Purpose: To determine and compare the antioxidant, antiproliferative and apoptotic effects of leaf infusions of Ilex laurina and Ilex paraguariensis in colon cancer cells. Methods: Antioxidant activity was determined by ORAC (Oxygen Radical Absorbance Capacity) and FRAP (Ferric Reducing Antioxidant Power). Cytotoxic ...
-
Antioxidant activity of the microalga Spirulina maxima
Miranda M.S.; Cintra R.G.; Barros S.B.M.; Mancini-Filho J.
1998-01-01
Spirulina maxima, which is used as a food additive, is a microalga rich in protein and other essential nutrients. Spirulina contains phenolic acids, tocopherols and ß-carotene which are known to exhibit antioxidant properties. The aim of the present study was to evaluate the antioxidant capacity of a Spirulina extract. The antioxidant activity of a methanolic extract of Spirulina was determined in vitro and in vivo. The in vitro antioxidant capacity was tested on a brain homogenate incubated ...
-
Poly(ethylene glycol-Prodrug Conjugates: Concept, Design, and Applications
Directory of Open Access Journals (Sweden)
Shashwat S. Banerjee
2012-01-01
Full Text Available Poly(ethylene glycol (PEG is the most widely used polymer in delivering anticancer drugs clinically. PEGylation (i.e., the covalent attachment of PEG of peptides proteins, drugs, and bioactives is known to enhance the aqueous solubility of hydrophobic drugs, prolong circulation time, minimize nonspecific uptake, and achieve specific tumor targetability through the enhanced permeability and retention effect. Numerous PEG-based therapeutics have been developed, and several have received market approval. A vast amount of clinical experience has been gained which has helped to design PEG prodrug conjugates with improved therapeutic efficacy and reduced systemic toxicity. However, more efforts in designing PEG-based prodrug conjugates are anticipated. In light of this, the current paper highlights the synthetic advances in PEG prodrug conjugation methodologies with varied bioactive components of clinical relevance. In addition, this paper discusses FDA-approved PEGylated delivery systems, their intended clinical applications, and formulations under clinical trials.
-
Recent advances in the construction of antibody-drug conjugates
Chudasama, Vijay; Maruani, Antoine; Caddick, Stephen
2016-02-01
Antibody-drug conjugates (ADCs) comprise antibodies covalently attached to highly potent drugs using a variety of conjugation technologies. As therapeutics, they combine the exquisite specificity of antibodies, enabling discrimination between healthy and diseased tissue, with the cell-killing ability of cytotoxic drugs. This powerful and exciting class of targeted therapy has shown considerable promise in the treatment of various cancers with two US Food and Drug Administration approved ADCs currently on the market (Adcetris and Kadcyla) and approximately 40 currently undergoing clinical evaluation. However, most of these ADCs exist as heterogeneous mixtures, which can result in a narrow therapeutic window and have major pharmacokinetic implications. In order for ADCs to deliver their full potential, sophisticated site-specific conjugation technologies to connect the drug to the antibody are vital. This Perspective discusses the strategies currently used for the site-specific construction of ADCs and appraises their merits and disadvantages.
-
Tubulin Inhibitor-Based Antibody-Drug Conjugates for Cancer Therapy
Directory of Open Access Journals (Sweden)
Hao Chen
2017-08-01
Full Text Available Antibody-drug conjugates (ADCs are a class of highly potent biopharmaceutical drugs generated by conjugating cytotoxic drugs with specific monoclonal antibodies through appropriate linkers. Specific antibodies used to guide potent warheads to tumor tissues can effectively reduce undesired side effects of the cytotoxic drugs. An in-depth understanding of antibodies, linkers, conjugation strategies, cytotoxic drugs, and their molecular targets has led to the successful development of several approved ADCs. These ADCs are powerful therapeutics for cancer treatment, enabling wider therapeutic windows, improved pharmacokinetic/pharmacodynamic properties, and enhanced efficacy. Since tubulin inhibitors are one of the most successful cytotoxic drugs in the ADC armamentarium, this review focuses on the progress in tubulin inhibitor-based ADCs, as well as lessons learned from the unsuccessful ADCs containing tubulin inhibitors. This review should be helpful to facilitate future development of new generations of tubulin inhibitor-based ADCs for cancer therapy.
-
Preparation of conjugated polymer suspensions by using ultrasonic atomizer
Energy Technology Data Exchange (ETDEWEB)
Tada, Kazuya, E-mail: tada@eng.u-hyogo.ac.jp; Onoda, Mitsuyoshi
2010-11-30
The electrophoretic deposition is a method useful to prepare conjugated polymer films for electronic devices. This method provides high material recovery rate on the substrate from the suspension, in contrast to the conventional spin-coating in which most of the material placed on the substrate is blown away. Although manual reprecipitation technique successfully yields suspensions of various conjugated polymers including polyfluorene derivatives, it is favorable to control the preparation process of suspensions. In this context, this paper reports preliminary results on the preparation of suspension of conjugated polymer by using an ultrasonic atomizer. While the resultant films do not show particular difference due to the preparation methods of the suspension, the electric current profiles during the electrophoretic deposition suggests that the ultrasonic atomization of polymer solution prior to be mixed with poor solvent results in smaller and less uniform colloidal particles than the conventional manual pouring method.
-
Shofian, Norshahida Mohamad; Hamid, Azizah Abdul; Osman, Azizah; Saari, Nazamid; Anwar, Farooq; Dek, Mohd Sabri Pak; Hairuddin, Muhammad Redzuan
2011-01-01
The effects of freeze-drying on antioxidant compounds and antioxidant activity of five tropical fruits, namely starfruit (Averrhoa carambola L.), mango (Mangifera indica L.), papaya (Carica papaya L.), muskmelon (Cucumis melo L.), and watermelon Citruluss lanatus (Thunb.) were investigated. Significant (p 0.05) change, however, observed in the ascorbic acid content of the fresh and freeze-dried fruits. Similarly, freeze-drying did not exert any considerable effect on β-carotene concentration of fruits, except for mango and watermelon, where significantly (p < 0.05) higher levels were detected in the fresh samples. The results of DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging and reducing power assays revealed that fresh samples of starfruit and mango had relatively higher antioxidant activity. In case of linoleic acid peroxidation inhibition measurement, a significant (p < 0.05) but random variation was recorded between the fresh and freeze-dried fruits. Overall, in comparison to β-carotene and ascorbic acid, a good correlation was established between the result of TPC and antioxidant assays, indicating that phenolics might have been the dominant compounds contributing towards the antioxidant activity of the fruits tested. PMID:21845104
-
Cancer Chemopreventive Ability of Conjugated Linolenic Acids
Directory of Open Access Journals (Sweden)
Kazuo Miyashita
2011-11-01
Full Text Available Conjugated fatty acids (CFA have received increased interest because of their beneficial effects on human health, including preventing cancer development. Conjugated linoleic acids (CLA are such CFA, and have been reviewed extensively for their multiple biological activities. In contrast to other types of CFAs including CLA that are found at low concentrations (less than 1% in natural products, conjugated linolenic acids (CLN are the only CFAs that occur in higher quantities in natural products. Some plant seeds contain a considerably high concentration of CLN (30 to 70 wt% lipid. Our research group has screened CLN from different plant seed oils to determine their cancer chemopreventive ability. This review describes the physiological functions of CLN isomers that occur in certain plant seeds. CLN are able to induce apoptosis through decrease of Bcl-2 protein in certain human cancer cell lines, increase expression of peroxisome proliferator-activated receptor (PPAR-γ, and up-regulate gene expression of p53. Findings in our preclinical animal studies have indicated that feeding with CLN resulted in inhibition of colorectal tumorigenesis through modulation of apoptosis and expression of PPARγ and p53. In this review, we summarize chemopreventive efficacy of CLN against cancer development, especially colorectal cancer.
-
Optical phase conjugation for time-domain undoing of dispersive self-phase-modulation effects
International Nuclear Information System (INIS)
Fisher, R.A.; Suydam, B.R.; Yevick, D.
1983-01-01
We show that the temporal distortion and spectral broadening of a pulse generated by the combined effects of group-velocity dispersion and self-phase modulation is removed by reflection of a cw-pumped, broadband, unity-reflecting Kerr-like optical phase conjugator followed by retraversal of the nonlinear medium. We also examine numerically the effects of finite linear loss in the material, of nonunity conjugate reflectivity, and of finite conjugator thickness
-
Hen Egg as an Antioxidant Food Commodity: A Review.
Nimalaratne, Chamila; Wu, Jianping
2015-09-24
Intake of antioxidants through diet is known to be important in reducing oxidative damage in cells and improving human health. Although eggs are known for their exceptional, nutritional quality, they are not generally considered as antioxidant foods. This review aims to establish the importance of eggs as an antioxidant food by summarizing the current knowledge on egg-derived antioxidants. Eggs have various natural occurring compounds including the proteins ovalbumin, ovotransferrin and lysozyme in egg white, as well as phosvitin, carotenoids and free aromatic amino acids in egg yolk. Some lipophilic antioxidants such as vitamin E, carotenoids, selenium, iodine and others can be transferred from feed into egg yolk to produce antioxidant-enriched eggs. The bioactivity of egg antioxidants can be affected by food processing, storage and gastrointestinal digestion. Generally thermal processing methods can promote loss of antioxidant properties in eggs due to oxidation and degradation, whereas gastrointestinal digestion enhances the antioxidant properties, due to the formation of new antioxidants (free amino acids and peptides). In summary, in addition to its well-known nutritional contribution to our diet, this review emphasizes the role of eggs as an important antioxidant food.
-
Antioxidant Vitamins and Trace Elements in Critical Illness.
Koekkoek, W A C Kristine; van Zanten, Arthur R H
2016-08-01
This comprehensive narrative review summarizes relevant antioxidant mechanisms, the antioxidant status, and effects of supplementation in critically ill patients for the most studied antioxidant vitamins A, C, and E and the enzyme cofactor trace elements selenium and zinc. Over the past 15 years, oxidative stress-mediated cell damage has been recognized to be fundamental to the pathophysiology of various critical illnesses such as acute respiratory distress syndrome, ischemia-reperfusion injury, and multiorgan dysfunction in sepsis. Related to these conditions, low plasma levels of antioxidant enzymes, vitamins, and trace elements have been frequently reported, and thus supplementation seems logical. However, low antioxidant plasma levels per se may not indicate low total body stores as critical illness may induce redistribution of antioxidants. Furthermore, low antioxidant levels may even be beneficial as pro-oxidants are essential in bacterial killing. The reviewed studies in critically ill patients show conflicting results. This may be due to different patient populations, study designs, timing, dosing regimens, and duration of the intervention and outcome measures evaluated. Therefore, at present, it remains unclear whether supplementation of antioxidant micronutrients has any clinical benefit in critically ill patients as some studies show clear benefits, whereas others demonstrate neutral outcomes and even harm. Combination therapy of antioxidants seems logical as they work in synergy and function as elements of the human antioxidant network. Further research should focus on defining the normal antioxidant status for critically ill patients and to study optimal supplement combinations either by nutrition enrichment or by enteral or parenteral pharmacological interventions. © 2016 American Society for Parenteral and Enteral Nutrition.
-
CSIR Research Space (South Africa)
Aderibigbe, BA
2015-01-01
Full Text Available Polyamidoamine conjugates containing curcumin and bisphosphonate were synthesized via a one-pot aqueous phase Michael addition reaction. In the design of the conjugate, bisphosphonate formed an integral part of the polymer carrier backbone. Curcumin...
-
Directory of Open Access Journals (Sweden)
Jiang X
2012-02-01
Full Text Available Danbo Yang1, Sang Van2, Yingyi Shu1, Xiaoqing Liu1, Yangfeng Ge1, Xinguo Jiang3, Yi Jin2, Lei Yu1,21Biomedical Engineering and Technology Institute, Institutes for Advanced Interdisciplinary Research, East China Normal University, Shanghai, People’s Republic of China; 2Biomedical Group, Nitto Denko Technical Corporation, CA, USA; 3School of Pharmacy, Fudan University, Shanghai, People’s Republic of ChinaAim: This work is intended to develop and evaluate a biopolymeric poly(L-γ-glutamyl-glutamine (PGG–docetaxel (DTX conjugate that can spontaneously self-assemble in aqueous solutions to become nanoparticles.Methods: DTX was covalently attached to hydrophilic PGG by direct esterification, and the conjugate was characterized by proton nuclear magnetic resonance spectroscopy, molecular weight gel permeation chromatography, solubility, size distribution and morphology, and hemolysis. Conjugated DTX was found to have 2000 times improved water solubility compared with free DTX. Dynamic light scattering, transmission electron microscopy, and atomic force microscopy revealed the particle size, distribution and morphology of the PGG–DTX conjugate. In addition, the conjugate was further tested for in vitro cytotoxicity and in vivo antitumor efficacy on the human non-small cell lung cancer cell line NCI-H460.Results: Conjugated DTX was found to have 2000 times improved water solubility compared with free DTX. The conjugate formed nanoparticles with an average diameter of 30 nm in spherical shape and unimodal particle size distribution. The conjugate exhibited about 2% hemolysis at 10 mg/mL, compared with 56% for Tween 80® at 0.4 mg/mL, and 33% for Cremophor EL® at 10 mg/mL. In addition, the conjugate was further tested for in vitro cytotoxicity and in vivo antitumor efficacy on the human non-small cell lung cancer cell line NCI-H460. As expected, conjugated DTX exhibited lower cytotoxicity compared to that of free DTX, in concentration
-
Peptide π-Electron Conjugates: Organic Electronics for Biology?
Ardoña, Herdeline Ann M; Tovar, John D
2015-12-16
Highly ordered arrays of π-conjugated molecules are often viewed as a prerequisite for effective charge-transporting materials. Studies involving these materials have traditionally focused on organic electronic devices, with more recent emphasis on biological systems. In order to facilitate the transition to biological environments, biomolecules that can promote hierarchical ordering and water solubility are often covalently appended to the π-electron unit. This review highlights recent work on π-conjugated systems bound to peptide moieties that exhibit self-assembly and aims to provide an overview on the development and emerging applications of peptide-based supramolecular π-electron systems.
-
Theory of periodic conjugate heat transfer
Zudin, Yuri B
2016-01-01
This book presents the theory of periodic conjugate heat transfer in detail. It offers a simplified description of the interaction between a solid body and a fluid as a boundary value problem of the heat conduction equation for the solid body.
-
Receptor-targeted aptamer-siRNA conjugate-directed transcriptional regulation of HIV-1
Zhou, Jiehua; Lazar, Daniel; Li, Haitang; Xia, Xin; Satheesan, Sangeetha; Charlins, Paige; O'Mealy, Denis; Akkina, Ramesh; Saayman, Sheena; Weinberg, Marc S.; Rossi, John J.; Morris, Kevin V.
2018-01-01
Gene-based therapies represent a promising therapeutic paradigm for the treatment of HIV-1, as they have the potential to maintain sustained viral inhibition with reduced treatment interventions. Such an option may represent a long-term treatment alternative to highly active antiretroviral therapy. Methods: We previously described a therapeutic approach, referred to as transcriptional gene silencing (TGS), whereby small noncoding RNAs directly inhibit the transcriptional activity of HIV-1 by targeting sites within the viral promoter, specifically the 5' long terminal repeat (LTR). TGS differs from traditional RNA interference (RNAi) in that it is characterized by concomitant silent-state epigenetic marks on histones and DNA. To deliver TGS-inducing RNAs, we developed functional RNA conjugates based on the previously reported dual function of the gp120 (A-1) aptamer conjugated to 27-mer Dicer-substrate anti-HIV-1 siRNA (dsiRNA), LTR-362. Results: We demonstrate here that high levels of processed guide RNAs localize to the nucleus in infected T lymphoblastoid CEM cell line and primary human CD4+ T-cells. Treatment of the aptamer-siRNA conjugates induced TGS with an ~10-fold suppression of viral p24 levels as measured at day 12 post infection. To explore the silencing efficacy of aptamer-siRNA conjugates in vivo, HIV-1-infected humanized NOD/SCID/IL2 rγnull mice (hu-NSG) were treated with the aptamer-siRNA conjugates. Systemic delivery of the A-1-stick-LTR-362 27-mer siRNA conjugates suppressed HIV-1 infection and protected CD4+ T cell levels in viremia hu-NSG mice. Principle conclusions: Collectively these data suggest that the gp120 aptamer-dsiRNA conjugate design is suitable for systemic delivery of small RNAs that can be used to suppress HIV-1. PMID:29556342
-
A Scanning Hologram Recorded by Phase Conjugate Property of Nonlinear Crystals
DEFF Research Database (Denmark)
Zi-Liang, Ping; Dalsgaard, Erik
1996-01-01
A methode of recording a scanning hologram with phase conjugate property of nonlinear crystal is provided. The principle of recording, setup and experiments are given.......A methode of recording a scanning hologram with phase conjugate property of nonlinear crystal is provided. The principle of recording, setup and experiments are given....
-
Conjugation of 3, 4-benzpyrene and 1, 2-benzanthracene with plant peptides
International Nuclear Information System (INIS)
Durmishidze, A.S.V.; Chrikishvili, D.I.; Devdariani, T.A.
1993-01-01
It is known that one of the main pathways in the biotransformation of certain xenobiotics is their conjugation with endogenous compounds of the plant cell. This work presents the results on the establishment of pathway of conjugation of BP and BA with endogenous compounds of the cell. Ten-day corn and pea seedlings, grown under sterile conditions, were incubated in aqueous solutions of [7,10 14 C]-3,4-benzpyrene and [9 14 C]-1,2-benzanthracene. The specific radioactivity of aqueous solutions of [7,10 14 C]-BP and [9 14 C]-BA was 2112·10 4 and 2006·10 4 Bq/ml, respectively. Individual highly radioactive conversion products of BP and BA were subjected to acid hydrolysis and qualitative analysis of the radioactive and nonradioactive compounds of the hydrolysates. An analysis of the nonradioactive components showed that they are peptides with various amino acid compositions. Thus, the investigated conversion products are conjugation products of hydroxy derivatives of BP and BA with endogenous cell peptides. The conversion products of BP and BA were investigated to detect conjugates with endogenous carbohydrates. Despite careful searches, the conjugates of interest could not be detected
-
Lin, Cheng-Yi; Peng, Hsiu-Hui; Chen, Mei-Hsiu; Sun, Jui-Sheng; Chang, Chih-Ju; Liu, Tse-Ying; Chen, Ming-Hong
2016-05-01
The formation of fibrous tissue is part of the natural healing response following a laminectomy. Severe scar tissue adhesion, known as epidural fibrosis, is a common cause of failed back surgery syndrome. In this study, by combining the advantages of drug treatment with a physical barrier, an ibuprofen-conjugated crosslinkable polygalacturonic acid and hyaluronic acid hydrogel was developed for epidural fibrosis prevention. Conjugation was confirmed and measured by 1D(1)H NMR spectroscopy.In vitroanalysis showed that the ibuprofen-conjugated polygalacturonic acid-hyaluronic acid hydrogel showed low cytotoxicity. In addition, the conjugated ibuprofen decreased prostaglandin E2production of the lipopolysaccharide-induced RAW264.7 cells. Histological data inin vivostudies indicated that the scar tissue adhesion of laminectomized male adult rats was reduced by the application of our ibuprofen-conjugated polygalacturonic acid-hyaluronic acid hydrogel. Its use also reduced the population of giant cells and collagen deposition of scar tissue without inducing extensive cell recruitment. The results of this study therefore suggest that the local delivery of ibuprofenviaa polygalacturonic acid-hyaluronic acid-based hydrogel reduces the possibility of epidural fibrosis. © The Author(s) 2016.
-
Martin, Julie; Péloquin, Katherine; Vachon, Marie-France; Duval, Michel; Sultan, Serge
The negative impact of paediatric cancer on parents is well known and is even greater when intensive treatments are used. This study aimed to describe how couples whose child has received a transplant for the treatment of leukaemia view conjugal resilience and to evaluate the role of we-ness as a precursor of conjugal adjustment. Four parental couples were interviewed. Interviews were analysed in two ways: inductive thematic analysis and rating of verbal content with the We-ness Coding Scale . Participants report that conjugal resilience involves the identification of the couple as a team and cohesion in the couple. Being a team generates certain collaborative interactions that lead to conjugal resilience. A sense of we-ness in parents is associated with fluctuation in the frequency of themes. Participants' vision of conjugal resilience introduced novel themes. The sense of we-ness facilitates cohesion and the process of conjugal resilience.
-
Analysis of Two Methods to Evaluate Antioxidants
Tomasina, Florencia; Carabio, Claudio; Celano, Laura; Thomson, Leonor
2012-01-01
This exercise is intended to introduce undergraduate biochemistry students to the analysis of antioxidants as a biotechnological tool. In addition, some statistical resources will also be used and discussed. Antioxidants play an important metabolic role, preventing oxidative stress-mediated cell and tissue injury. Knowing the antioxidant content…
-
Flavonoid, hesperidine, total phenolic contents and antioxidant ...
African Journals Online (AJOL)
Additionally, the antioxidant activities were also determined by ferric reducing antioxidant power (FRAP) and 1,1-diphenyl-2-picryl hydrazyl (DPPH) radical scavenging activity. C. hystrix had the highest flavonoid and total phenolic contents while C. aurantifolia had the highest hesperidine content. The antioxidant activity of ...
-
Experimental demonstration of continuous variable cloning with phase-conjugate inputs
DEFF Research Database (Denmark)
Sabuncu, Metin; Andersen, Ulrik Lund; Leuchs, G.
2007-01-01
We report the first experimental demonstration of continuous variable cloning of phase-conjugate coherent states as proposed by Cerf and Iblisdir [Phys. Rev. Lett. 87, 247903 (2001)]. In contrast to this proposal, the cloning transformation is accomplished using only linear optical components......, homodyne detection, and feedforward. As a result of combining phase conjugation with a joint measurement strategy, superior cloning is demonstrated with cloning fidelities reaching 89%....
-
Directory of Open Access Journals (Sweden)
Hoseinpouran Manuchehr
2015-10-01
Full Text Available Objective: The aim of this study is to assess the antioxidant properties of onion on biochemical serum factors, antioxidants and testicular tissues in Wistar rats after consuming tartrazine. Materials and Methods: Forty male Wistar were divided into four groups of 10. The first group was used as the control group and were given only water without additives, group 2 were given tartrazine, group 3 were given tartrazine plus onion juice and the fourth group which was given only onion juice through gastric gavage. The experiment was conducted for 60 days, then the antioxidant activities superoxide dismutase (SOD, catalase (CAT, glutathione peroxidase (GPx and biochemical parameters namely high density lipoprotein (HDL, low density lipoprotein (LDL and testosterone together with the histopathological studies (sperm count and testicular weight were measured. Results: Tartrazine caused a decrease in the activity of antioxidant enzymes (SOD, CAT, GPX and a decrease in the level of testosterone and HDL and also a decrease in sperm count and testicular tissue weight. Tartrazine caused an increase in the LDL levels. Conclusion: Results showed that consumption of tartrazine is associated with production of free radicals and in turn causes significant decrease in antioxidant activities and biochemical serum factors which damage the cellular compartments of the testis. Onion as an antioxidant in this study reduces the damaging effects of tartrazine on the enzymatic activities of antioxidant and biochemical serum factors.
-
Ti-Catalyzed Hydroamination for the Synthesis of Amine-Containing π-Conjugated Materials.
Hao, Han; Thompson, Kyle A; Hudson, Zachary M; Schafer, Laurel L
2018-04-11
A series of conjugated enamines were prepared by Ti catalyzed anti-Markovnikov hydroamination. The synthetic route is efficient with yields of up to 94 % and the 100 % atom efficiency of the reaction means that these products are easily isolated and purified. Due to the extended conjugated system, the enamine tautomers were observed exclusively in both solid and solution phases, as determined by X-ray crystallography and NMR spectroscopy. These new conjugated molecules, with N incorporated into the backbone, show interesting photophysical properties including photo-luminescent quantum yields of up to 0.26. Notably, through the incorporation of B to give a donor-acceptor π-conjugated system, a redshift of approximately 100 nm is observed for the emission maximum along with the anticipated solvatochromic shifts. © 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
-
Antioxidants: Characterization, natural sources, extraction and analysis.
Oroian, Mircea; Escriche, Isabel
2015-08-01
Recently many review papers regarding antioxidants from different sources and different extraction and quantification procedures have been published. However none of them has all the information regarding antioxidants (chemistry, sources, extraction and quantification). This article tries to take a different perspective on antioxidants for the new researcher involved in this field. Antioxidants from fruit, vegetables and beverages play an important role in human health, for example preventing cancer and cardiovascular diseases, and lowering the incidence of different diseases. In this paper the main classes of antioxidants are presented: vitamins, carotenoids and polyphenols. Recently, many analytical methodologies involving diverse instrumental techniques have been developed for the extraction, separation, identification and quantification of these compounds. Antioxidants have been quantified by different researchers using one or more of these methods: in vivo, in vitro, electrochemical, chemiluminescent, electron spin resonance, chromatography, capillary electrophoresis, nuclear magnetic resonance, near infrared spectroscopy and mass spectrometry methods. Copyright © 2015. Published by Elsevier Ltd.
-
Conjugated polymers developed from alkynes
Institute of Scientific and Technical Information of China (English)
Yajing Liu; Jacky W.Y.Lam; Ben Zhong Tang
2015-01-01
The numerous merits of conjugated polymers(CPs) have encouraged scientists to develop a variety of synthetic routes to CPs with diverse structures and functionalities. Among the large scope of substrates,alkyne plays an important role in constructing polymers with conjugated backbones. In addition to some well-developed reactions including Glaser–Hay and Sonogashira coupling, azide/thiol-yne click reaction and cyclotrimerization, some novel alkyne-based reactions have also been explored such as oxidative polycoupling, decarbonylative polycoupling and multicomponent tandem polymerizations. his review focuses on the recent progress on the synthetic methodology of CPs in the last ive years using monomers with two or more triple bonds and some of their high-technological applications. Selected examples of materials properties of these CPs are given in this review, such as luorescence response to chemical or physical stimuli, magnetism, white light emission, cell imaging and bioprobing. Finally, a short perspective is raised in regard to the outlook of the preparation methodologies, functionalities as well as potential applications of CPs in the future.
-
Subgap Absorption in Conjugated Polymers
Sinclair, M.; Seager, C. H.; McBranch, D.; Heeger, A. J; Baker, G. L.
1991-01-01
Along with X{sup (3)}, the magnitude of the optical absorption in the transparent window below the principal absorption edge is an important parameter which will ultimately determine the utility of conjugated polymers in active integrated optical devices. With an absorptance sensitivity of materials. We have used PDS to measure the optical absorption spectra of the conjugated polymers poly(1,4-phenylene-vinylene) (and derivitives) and polydiacetylene-4BCMU in the spectral region from 0.55 eV to 3 eV. Our spectra show that the shape of the absorption edge varies considerably from polymer to polymer, with polydiacetylene-4BCMU having the steepest absorption edge. The minimum absorption coefficients measured varied somewhat with sample age and quality, but were typically in the range 1 cm{sup {minus}1} to 10 cm{sup {minus}1}. In the region below 1 eV, overtones of C-H stretching modes were observed, indicating that further improvements in transparency in this spectral region might be achieved via deuteration of fluorination.
-
π-Clamp-mediated cysteine conjugation
Zhang, Chi; Welborn, Matthew; Zhu, Tianyu; Yang, Nicole J.; Santos, Michael S.; van Voorhis, Troy; Pentelute, Bradley L.
2016-02-01
Site-selective functionalization of complex molecules is one of the most significant challenges in chemistry. Typically, protecting groups or catalysts must be used to enable the selective modification of one site among many that are similarly reactive, and general strategies that selectively tune the local chemical environment around a target site are rare. Here, we show a four-amino-acid sequence (Phe-Cys-Pro-Phe), which we call the ‘π-clamp’, that tunes the reactivity of its cysteine thiol for site-selective conjugation with perfluoroaromatic reagents. We use the π-clamp to selectively modify one cysteine site in proteins containing multiple endogenous cysteine residues. These examples include antibodies and cysteine-based enzymes that would be difficult to modify selectively using standard cysteine-based methods. Antibodies modified using the π-clamp retained binding affinity to their targets, enabling the synthesis of site-specific antibody-drug conjugates for selective killing of HER2-positive breast cancer cells. The π-clamp is an unexpected approach to mediate site-selective chemistry and provides new avenues to modify biomolecules for research and therapeutics.
-
Protection against ionizing radiation by antioxidant nutrients and phytochemicals
International Nuclear Information System (INIS)
Weiss, Joseph F.; Landauer, Michael R.
2003-01-01
The potential of antioxidants to reduce the cellular damage induced by ionizing radiation has been studied in animal models for more than 50 years. The application of antioxidant radioprotectors to various human exposure situations has not been extensive although it is generally accepted that endogenous antioxidants, such as cellular non-protein thiols and antioxidant enzymes, provide some degree of protection. This review focuses on the radioprotective efficacy of naturally occurring antioxidants, specifically antioxidant nutrients and phytochemicals, and how they might influence various endpoints of radiation damage. Results from animal experiments indicate that antioxidant nutrients, such as vitamin E and selenium compounds, are protective against lethality and other radiation effects but to a lesser degree than most synthetic protectors. Some antioxidant nutrients and phytochemicals have the advantage of low toxicity although they are generally protective when administered at pharmacological doses. Naturally occurring antioxidants also may provide an extended window of protection against low-dose, low-dose-rate irradiation, including therapeutic potential when administered after irradiation. A number of phytochemicals, including caffeine, genistein, and melatonin, have multiple physiological effects, as well as antioxidant activity, which result in radioprotection in vivo. Many antioxidant nutrients and phytochemicals have antimutagenic properties, and their modulation of long-term radiation effects, such as cancer, needs further examination. In addition, further studies are required to determine the potential value of specific antioxidant nutrients and phytochemicals during radiotherapy for cancer
-
New Conjugacy Conditions and Related Nonlinear Conjugate Gradient Methods
International Nuclear Information System (INIS)
Dai, Y.-H.; Liao, L.-Z.
2001-01-01
Conjugate gradient methods are a class of important methods for unconstrained optimization, especially when the dimension is large. This paper proposes a new conjugacy condition, which considers an inexact line search scheme but reduces to the old one if the line search is exact. Based on the new conjugacy condition, two nonlinear conjugate gradient methods are constructed. Convergence analysis for the two methods is provided. Our numerical results show that one of the methods is very efficient for the given test problems
-
International Nuclear Information System (INIS)
Chen, G.S.
1997-01-01
We apply and compare the preconditioned generalized conjugate gradient methods to solve the linear system equation that arises in the two-dimensional neutron and photon transport equation in this paper. Several subroutines are developed on the basis of preconditioned generalized conjugate gradient methods for time-independent, two-dimensional neutron and photon transport equation in the transport theory. These generalized conjugate gradient methods are used. TFQMR (transpose free quasi-minimal residual algorithm), CGS (conjuage gradient square algorithm), Bi-CGSTAB (bi-conjugate gradient stabilized algorithm) and QMRCGSTAB (quasi-minimal residual variant of bi-conjugate gradient stabilized algorithm). These sub-routines are connected to computer program DORT. Several problems are tested on a personal computer with Intel Pentium CPU. (author)
-
Synthesis and evaluation of 99mTc/99Tc-MAG3-biotin conjugates for antibody pretargeting strategies
International Nuclear Information System (INIS)
Gog, Frank B. van; Visser, Gerard W.M.; Gowrising, Radjish W.A.; Snow, Gordon B.; Dongen, Guus A.M.S. van
1998-01-01
Four 99m Tc-MAG3-biotin conjugates were synthesized to determine their potential use in antibody pretargeting strategies for radioimmunoscintigraphy (RIS). To use these 99m Tc-MAG3-biotin conjugates as model compounds for 186 Re-MAG3-biotin conjugates for radioimmunotherapy (RIT), nanomolar amounts of 99 Tc were added as carrier to 99m Tc. The biotin derivatives used for the preparation of the conjugates - biocytin, biotin hydrazide, biotinyl-piperazine, and biotinyl-diaminosuccinic acid - differed at the site that is regarded to be susceptible to hydrolysis by biotinidase present in human plasma. All four conjugates were produced with high radiochemical purity, were stable in PBS, and demonstrated full binding capacity to streptavidin. The 99m Tc/ 99 Tc-MAG3-labeled biotinyl-piperazine and biotinyl-diaminosuccinic acid conjugates were stable in mouse as well as human plasma, whereas the corresponding biocytin and biotin hydrazide conjugates were rapidly degraded. The biodistribution in nude mice at 30 min after injection was similar for all conjugates, and a rapid blood clearance and high intestinal excretion were both observed. It is concluded that the metabolic routing of a conjugate containing biotin and MAG3 is dominated by these two moieties. For this reason, MAG3-biotin conjugates do not seem suited for pretargeted RIT, for which quantitative and fast renal excretion is a prerequisite to minimize radiation toxicity. However, in a pretargeted RIS approach the 99m Tc-MAG3-biotin conjugates might have potential
-
Particle-in-a-box model of exciton absorption and electroabsorption in conjugated polymers
Pedersen, Thomas G.
2000-12-01
The recently proposed particle-in-a-box model of one-dimensional excitons in conjugated polymers is applied in calculations of optical absorption and electroabsorption spectra. It is demonstrated that for polymers of long conjugation length a superposition of single exciton resonances produces a line shape characterized by a square-root singularity in agreement with experimental spectra near the absorption edge. The effects of finite conjugation length on both absorption and electroabsorption spectra are analyzed.
-
Antioxidant Activity of Hawaiian Marine Algae
Directory of Open Access Journals (Sweden)
Anthony D. Wright
2012-02-01
Full Text Available Marine algae are known to contain a wide variety of bioactive compounds, many of which have commercial applications in pharmaceutical, medical, cosmetic, nutraceutical, food and agricultural industries. Natural antioxidants, found in many algae, are important bioactive compounds that play an important role against various diseases and ageing processes through protection of cells from oxidative damage. In this respect, relatively little is known about the bioactivity of Hawaiian algae that could be a potential natural source of such antioxidants. The total antioxidant activity of organic extracts of 37 algal samples, comprising of 30 species of Hawaiian algae from 27 different genera was determined. The activity was determined by employing the FRAP (Ferric Reducing Antioxidant Power assays. Of the algae tested, the extract of Turbinaria ornata was found to be the most active. Bioassay-guided fractionation of this extract led to the isolation of a variety of different carotenoids as the active principles. The major bioactive antioxidant compound was identified as the carotenoid fucoxanthin. These results show, for the first time, that numerous Hawaiian algae exhibit significant antioxidant activity, a property that could lead to their application in one of many useful healthcare or related products as well as in chemoprevention of a variety of diseases including cancer.
-
Butylated caffeic acid: An efficient novel antioxidant
Directory of Open Access Journals (Sweden)
G. Shi
2017-09-01
Full Text Available A novel antioxidant, butylated caffeic acid (BCA was rationally designed by adding a tert-butyl group to caffeic acid, which was synthesized at a high yield (36.2% from 2-methoxy-4-methylphenol by a four-step reaction including Friedel-Crafts alkylation, bromine oxidation, ether bond hydrolysis and Knoevenagel condensation. Its antioxidant capacity was much stronger than common commercial antioxidant tert-butyl hydroquinone (TBHQ and its mother compound, caffeic acid, in both rancimat and deep frying tests. When investigated via the DPPH method, the antioxidant capacity of BCA was almost equal to TBHQ, but lower than caffeic acid. BCA could be a potentially strong antioxidant, especially for food processing at high temperatures such as deep frying and baking.
-
Butylated caffeic acid: An efficient novel antioxidant
International Nuclear Information System (INIS)
Shi, G.; Liao, X.; Olajide, T.M.; Liu, J.; Jiang, X.; Weng, X.
2017-01-01
A novel antioxidant, butylated caffeic acid (BCA) was rationally designed by adding a tert-butyl group to caffeic acid, which was synthesized at a high yield (36.2%) from 2-methoxy-4-methylphenol (1) by a four-step reaction including Friedel-Crafts alkylation, bromine oxidation, ether bond hydrolysis and Knoevenagel condensation. Its antioxidant capacity was much stronger than common commercial antioxidant tert-butyl hydroquinone (TBHQ) and its mother compound, caffeic acid, in both rancimat and deep frying tests. When investigated via the DPPH method, the antioxidant capacity of BCA was almost equal to TBHQ, but lower than caffeic acid. BCA could be a potentially strong antioxidant, especially for food processing at high temperatures such as deep frying and baking. [es
-
Rhenium 188 labelling of peptide conjugates
International Nuclear Information System (INIS)
Melendez-Alafort, Laura
2001-01-01
Many human tumours express high levels, of somatostatin receptors. In order to make possible a radiotherapeutic treatment of this kind for tumour a series of somatostatin analogues that can tightly chelate beta emitting isotopes have been developed in recent years. The work carried out for this thesis has been aimed towards development of a new therapeutic radiopharmaceutical for treatment of somatostatin receptor positive tumours. The first chapters describe work with technetium-99m to establish the labelling and analytical conditions for a somatostatin analogue, [Tyr 3 ]-octreotide (TOC), as a precursor to undertaking labelling studies with the beta emitter rhenium-188. 6-Hydrazinopyridine-3-carboxylic acid (HYNIC) was conjugated to TOC and labelled with 99m using different coligands. Then the stability, receptor binding and biodistribution of each complex were assessed. 99m Tc-HYNIC-TOC using EDDA as coligand showed the best characteristics, and was superior for tumour imaging in humans than the commercially available 111 In-DTPA-octreotide. The conditions for labelling the HYNIC-TOC conjugate with 188 Re were then optimised using tricine as a co-ligand. A labelling yield of ∼80% was achieved. After purification however, the stability of the complex was low. The use of other coligand systems which had proved useful for 99m Tc labelling was explored, but yields were very poor. Other chelators such as diethylenetriamine pentaacetic acid (DTPA), dimercaptosuccinic acid (DMSA) and mercaptoacetyltriglycine (MAG 3 ) were studied as potential co-ligand agents to label the HYNIC-TOC conjugate with 188 Re but, again low yields of the labelled peptide complexes were achieved. A novel 188 Re-HYNIC complex was prepared in high yields using N-N-disubstituted dithiocarbamates as coligands. However to date, the specific activities achieved with this system are relatively low. The use of the [ 99m Tc(CO) 3 (H 2 O) 3 ] complex to label the HYNIC-TOC conjugate was investigated
-
Total antioxidant content of alternatives to refined sugar.
Phillips, Katherine M; Carlsen, Monica H; Blomhoff, Rune
2009-01-01
Oxidative damage is implicated in the etiology of cancer, cardiovascular disease, and other degenerative disorders. Recent nutritional research has focused on the antioxidant potential of foods, while current dietary recommendations are to increase the intake of antioxidant-rich foods rather than supplement specific nutrients. Many alternatives to refined sugar are available, including raw cane sugar, plant saps/syrups (eg, maple syrup, agave nectar), molasses, honey, and fruit sugars (eg, date sugar). Unrefined sweeteners were hypothesized to contain higher levels of antioxidants, similar to the contrast between whole and refined grain products. To compare the total antioxidant content of natural sweeteners as alternatives to refined sugar. The ferric-reducing ability of plasma (FRAP) assay was used to estimate total antioxidant capacity. Major brands of 12 types of sweeteners as well as refined white sugar and corn syrup were sampled from retail outlets in the United States. Substantial differences in total antioxidant content of different sweeteners were found. Refined sugar, corn syrup, and agave nectar contained minimal antioxidant activity (sugar had a higher FRAP (0.1 mmol/100 g). Dark and blackstrap molasses had the highest FRAP (4.6 to 4.9 mmol/100 g), while maple syrup, brown sugar, and honey showed intermediate antioxidant capacity (0.2 to 0.7 mmol FRAP/100 g). Based on an average intake of 130 g/day refined sugars and the antioxidant activity measured in typical diets, substituting alternative sweeteners could increase antioxidant intake an average of 2.6 mmol/day, similar to the amount found in a serving of berries or nuts. Many readily available alternatives to refined sugar offer the potential benefit of antioxidant activity.
-
Nano-assembly of nanodiamonds by conjugation to actin filaments.
Bradac, Carlo; Say, Jana M; Rastogi, Ishan D; Cordina, Nicole M; Volz, Thomas; Brown, Louise J
2016-03-01
Fluorescent nanodiamonds (NDs) are remarkable objects. They possess unique mechanical and optical properties combined with high surface areas and controllable surface reactivity. They are non-toxic and hence suited for use in biological environments. NDs are also readily available and commercially inexpensive. Here, the exceptional capability of controlling and tailoring their surface chemistry is demonstrated. Small, bright diamond nanocrystals (size ˜30 nm) are conjugated to protein filaments of actin (length ˜3-7 µm). The conjugation to actin filaments is extremely selective and highly target-specific. These unique features, together with the relative simplicity of the conjugation-targeting method, make functionalised nanodiamonds a powerful and versatile platform in biomedicine and quantum nanotechnologies. Applications ranging from using NDs as superior biological markers to, potentially, developing novel bottom-up approaches for the fabrication of hybrid quantum devices that would bridge across the bio/solid-state interface are presented and discussed. © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
-
Progress towards meningitis prevention in the conjugate vaccines era
Directory of Open Access Journals (Sweden)
Cristina Aparecida Borges Laval
Full Text Available Acute bacterial meningitis is an important cause of morbidity and mortality among children less than five years old. Haemophilus influenzae, Streptococcus pneumoniae and Neisseria meningitidis are the most important agents of bacterial meningitis in developing countries. The development of the conjugate vaccines in the beginning of the 90's, especially type b H. influenzae (Hib, and more recently the heptavalent pneumococcal and the serogroup C meningococcal vaccines, have contributed directly to changes in the epidemiological profile of these invasive diseases (direct effect and of their carriage status (indirect effect. We review the impact of the Hib conjugate vaccine in Latin American countries, where this vaccine has been implemented, and the potential of pneumococcal and meningococcal conjugate vaccines for the reduction of meningitis worldwide. We also address constraints for the development and delivery of these vaccines and review new candidate state-of-the-art vaccines. The greatest challenge, undoubtedly, is to implement these vaccines worldwide, especially in the developing regions.
-
Experiments with conjugate gradient algorithms for homotopy curve tracking
Irani, Kashmira M.; Ribbens, Calvin J.; Watson, Layne T.; Kamat, Manohar P.; Walker, Homer F.
1991-01-01
There are algorithms for finding zeros or fixed points of nonlinear systems of equations that are globally convergent for almost all starting points, i.e., with probability one. The essence of all such algorithms is the construction of an appropriate homotopy map and then tracking some smooth curve in the zero set of this homotopy map. HOMPACK is a mathematical software package implementing globally convergent homotopy algorithms with three different techniques for tracking a homotopy zero curve, and has separate routines for dense and sparse Jacobian matrices. The HOMPACK algorithms for sparse Jacobian matrices use a preconditioned conjugate gradient algorithm for the computation of the kernel of the homotopy Jacobian matrix, a required linear algebra step for homotopy curve tracking. Here, variants of the conjugate gradient algorithm are implemented in the context of homotopy curve tracking and compared with Craig's preconditioned conjugate gradient method used in HOMPACK. The test problems used include actual large scale, sparse structural mechanics problems.
-
Pomeron-Quark Coupling from Charge Conjugation Invariance
International Nuclear Information System (INIS)
Zhou Lijuan; Wu Qing; Ma Weixing; Gu Yunting
2006-01-01
Based on the charge conjugation invariance and the vacuum property of the Pomeron, we point out that the commonly used vector vertex of the Pomeron coupling to quark is incorrect since it contradicts with the Pomeron property. We also claim that the soft Pomeron could be a tensor glueball ξ(2230) with quantum numbers I G J PC = 0 + 2 ++ and total decay width Γ tot ≅100 MeV, which lies on the soft Pomeron trajectory α p = 1.08+0.20t. Therefore, the coupling vertex of the soft Pomeron to quark should be tensorial which is invariant under the charge conjugation and can explain why the inadequate vector coupling, γ μ , of the soft Pomeron to quark is successful in dealing with Pomeron physics.